��Ŀ����
���л�ѧ��Ӧ���ܲ���������̼���壺
��C+O2
CO2 ��CaCO3+2HCl�TCaCl2+CO2��+H2O ��2CO+O2
2CO2 ��CO+CuO
Cu+2CO2��
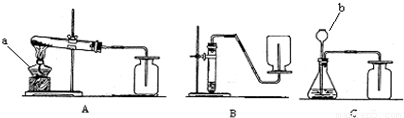
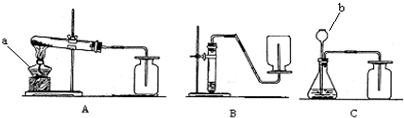
��1�����ϻ�ѧ��Ӧ�У���������ʵ������ȡ������̼����ķ�Ӧ��______��
��2����������ѡ��Ļ�ѧ��Ӧ����ͼ��ʾ�п�����ȡ������̼�����װ����______�����ø�װ�û�������ȡ��һ��������______����Ӧ�Ļ�ѧ����ʽΪ______��
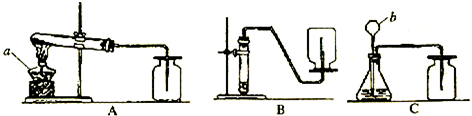
��3��ʵ������ȡ�������Ҫ�����У��ٹ̶�װ�� ��װ��ҩƷ �ۼ��� �ܼ��װ�õ������Ԣ��ռ����壮����ȷ�IJ���˳����______��
��4��д������װ���б�����������ƣ�a______��b______��
��5��ijͬѧ��Aװ����ȡ����������7.9g��KMnO4���ȷֽ⣬�ܵõ���״���µ�����������������״�����������ܶ�ԼΪ1.43g/L��������������һλС����
��C+O2
| ||
| ||
| ||
��1�����ϻ�ѧ��Ӧ�У���������ʵ������ȡ������̼����ķ�Ӧ��______��
��2����������ѡ��Ļ�ѧ��Ӧ����ͼ��ʾ�п�����ȡ������̼�����װ����______�����ø�װ�û�������ȡ��һ��������______����Ӧ�Ļ�ѧ����ʽΪ______��

��3��ʵ������ȡ�������Ҫ�����У��ٹ̶�װ�� ��װ��ҩƷ �ۼ��� �ܼ��װ�õ������Ԣ��ռ����壮����ȷ�IJ���˳����______��
��4��д������װ���б�����������ƣ�a______��b______��
��5��ijͬѧ��Aװ����ȡ����������7.9g��KMnO4���ȷֽ⣬�ܵõ���״���µ�����������������״�����������ܶ�ԼΪ1.43g/L��������������һλС����
��1����ʵ��������ȡ���岻��ʹ��������Ϊԭ�ϣ��٢ۢ�������Ӧ��ʹ����������Ϊ��Ӧ�����ʹ�ƵõĶ�����̼�л��вμӷ�Ӧ�����壬�ռ����������Ķ�����̼��
��2����ȡ������̼��̼��ƺ�ϡ���ᣬ�ǹ����Һ�巴Ӧ��ȡ���壬����Ҫ���ȣ����Դ�BC��ѡ������Ϊ������̼�ܶȱȿ�����ֻ���������ſ������ռ�����ѡCװ�ã���װ�û�������ȡ��������Ӧ���ǹ������⣬��������������ˮ����Ӧ�����Ƕ����������������ù۲취��ƽ��
��3��ʵ������ȡ�������Ҫ���裺�ȼ��װ�õ������ԣ�װҩƷ���̶������ȣ��ռ���
��4����dz������������ƺ���;��
��5������������������Ϊx��
2KMnO4
K2MnO4+MnO2+O2��
316 32
7.9g x
���ݣ�
=
��� x=0.8 g
��״�������������Ϊ��
��0.6 L
�ʴ�Ϊ����1���ڣ���2��C O2 2H2O2
2H2O+O2������3���ܢڢ٢ۢݣ���4���ƾ��ƣ� ����©���� ��5��0.6 L
��2����ȡ������̼��̼��ƺ�ϡ���ᣬ�ǹ����Һ�巴Ӧ��ȡ���壬����Ҫ���ȣ����Դ�BC��ѡ������Ϊ������̼�ܶȱȿ�����ֻ���������ſ������ռ�����ѡCװ�ã���װ�û�������ȡ��������Ӧ���ǹ������⣬��������������ˮ����Ӧ�����Ƕ����������������ù۲취��ƽ��
��3��ʵ������ȡ�������Ҫ���裺�ȼ��װ�õ������ԣ�װҩƷ���̶������ȣ��ռ���
��4����dz������������ƺ���;��
��5������������������Ϊx��
2KMnO4
| ||
316 32
7.9g x
���ݣ�
| 316 |
| 32 |
| 7.9g |
| x |
��״�������������Ϊ��
| 0.8g |
| 1.43g/L |
�ʴ�Ϊ����1���ڣ���2��C O2 2H2O2
| ||

��ϰ��ϵ�д�
�����Ŀ

 CO2
CO2 Cu+CO2
Cu+CO2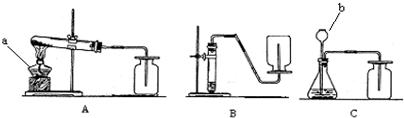
 CO2
CO2 2CO2
2CO2 Cu+CO2
Cu+CO2