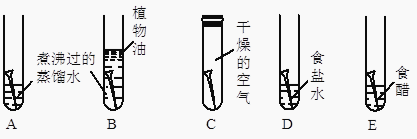��Ŀ����
����Ŀ��̼��̼�Ļ���������Ȼ��㷺���ڣ���ش�
��1�����ʯ��ʯī�����ֳ�����̼���ʣ��������������ܴ��ԭ���� ��
��2���������й�C��CO��CO2�������ʵ�˵���� ���������ʶ�����̼Ԫ�أ�������ȼ��
��CO��CO2����û����ɫ��û����ζ������
��CO2�����ڹ�����ã�CO�������˹�����
��CO2�ܲ�������ЧӦ��CO����ѪҺ�е�Ѫ�쵰��������ж�
��CO2���������CO������ȼ��
����˵������ȷ����
A.�٢ڢ�
B.�ڢۢ�
C.�ڢܢ�
D.�٢ۢ�
��3�������dz��õ�ʵ��װ��ͼ�� 
��װ��B��װ��C���������ƶ�����̼������װ��C���ŵ�����
��CO2ͨ��Eװ���е������� �� ��Ӧ����ʽΪ
������Dװ���ռ�H2 �� ��H2Ӧ�����a����b������ͨ�룮
��д��ʵ������Aװ����ȡ�����Ļ�ѧ����ʽ ��
��4��ʵ���ҳ���ʯ��ʯ��ϡ������ȡ������̼��ȡ��̼���80%��ʯ��ʯ12.5g������ϡ������ȫ��Ӧ��ʯ��ʯ�е����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ���������ɶ�����̼��������
���𰸡�
��1��̼ԭ�����з�ʽ��ͬ
��2��C
��3�����Ʒ�Ӧ�ķ�����ֹͣ������ʯ��ˮ����ǣ�Ca��OH��2+CO2=CaCO3��+H2O��b��2KMnO4 ![]() K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��
��4���⣺��̼���80%��ʯ��ʯ12.5g�к�̼��Ƶ�����Ϊ12.5g��80%=10g��
�����ɶ�����̼������Ϊx��
CaCO3 | +2HCl=CaCl2+H2O+ | CO2�� |
100 | 44 | |
10g | x |
![]()
x=4.4g��
�����ɶ�����̼������Ϊ4.4g
���������⣺��1�����ڽ��ʯ��̼ԭ�������ǿռ���״�ṹ��ʯī��̼ԭ�������Dz�״�ṹ�����ʯ��ʯī���ڲ�̼ԭ�ӵ����з�ʽ��ͬ�����¶��������������ʲ���ܴ�2�����������ʶ�����̼Ԫ�أ�����CO2��ȼ��Ҳ��֧��ȼ�գ��ʴ���CO��CO2����û����ɫ��û����ζ�����壬����ȷ����CO2�����ڹ�����ã�������Ϊ������ϣ��ɱ��������˹����꣬�ʴ���CO2�ܲ�������ЧӦ��CO����ѪҺ�е�Ѫ�쵰��������ж�������ȷ����CO2��ȼ��Ҳ��֧��ȼ�գ����������CO���п�ȼ�ԣ�������ȼ�ϣ�����ȷ�� ��ѡ��C����3��������װ��C����ͨ�������Һ��ķ������������Ʒ�Ӧ�ķ�����ֹͣ����Bװ����ҩƷ������ܷ��룬һ�������Ͳ��ܿ��ƣ���Cװ�õ��ŵ��ǣ����Ʒ�Ӧ�ķ�����ֹͣ���ڶ�����̼���������Ʒ�����Ӧ����̼��Ƴ�����ˮ����˿ɹ۲쵽������ʯ��ˮ����ǣ���ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��������Fװ���ռ�H2 �� �����������ܶȱȿ���С��������Ӧ��b��ͨ�룻�ܸ�����طֽ����������ء��������̺���������ѧ����ʽΪ��2KMnO4 ![]() K2MnO4+MnO2+O2����
K2MnO4+MnO2+O2����
���Դ��ǣ���1��̼ԭ�����з�ʽ��ͬ����2��C����3���ٿ��Ʒ�Ӧ�ķ�����ֹͣ���ڳ���ʯ��ˮ����ǣ�Ca��OH��2+CO2=CaCO3��+H2O����b����2KMnO4 ![]() K2MnO4+MnO2+O2������4��4.4g��
K2MnO4+MnO2+O2������4��4.4g��
�����㾫�������ڱ��⿼��Ķ�����̼�Ļ�ѧ���ʺ�һ����̼�����ʣ���Ҫ�˽⻯ѧ���ʣ�һ������²���ȼ��,Ҳ��֧��ȼ�գ����ܹ�����������ˮ��Ӧ����̼���ʹ�����ʯ��ˮ����ǣ������ȵ�̼��Ӧ��һ����̼���������ʣ���ɫ����ζ�����壬�ܶȱȿ�����С��������ˮ��һ����̼�Ļ�ѧ���ʣ���ȼ�Ժͻ�ԭ�Բ��ܵó���ȷ�𰸣�