��Ŀ����
��2013?��ɽ��һģ��ѧϰ��ѧ֪ʶ��Ŀ��֮һ�Ƿ����ͽ��ʵ�����⣮
��1���³�����ԷŸ�����̿��ȥ����ζ�����л���̿��
��2��������������ȼ�ϣ���ԭ����
��3��ʳƷ���ʳ�������հ�װ����䱣��������հ�װ��Ŀ����
��4�����ܷ����õķ������ƽԭ������ԭʱ����װ�����𣬴��۽Ƕȷ�������Ϊ
��5���⻯�ƣ�CaH2����������ڵ�ɽ��Ա����Դ�ṩ��������ˮ��Ӧ�����������ƺ���������Ӧ�Ļ�ѧ����ʽΪ
��1���³�����ԷŸ�����̿��ȥ����ζ�����л���̿��
����
����
���ã���2��������������ȼ�ϣ���ԭ����
2H2+O2
2H2O
| ||
2H2+O2
2H2O
���û�ѧ����ʽ��ʾ����
| ||
��3��ʳƷ���ʳ�������հ�װ����䱣��������հ�װ��Ŀ����
ʹʳƷ�����������������������ֹʳƷ��������
ʹʳƷ�����������������������ֹʳƷ��������
����4�����ܷ����õķ������ƽԭ������ԭʱ����װ�����𣬴��۽Ƕȷ�������Ϊ
���ڵ�������Ӽ�����
���ڵ�������Ӽ�����
����5���⻯�ƣ�CaH2����������ڵ�ɽ��Ա����Դ�ṩ��������ˮ��Ӧ�����������ƺ���������Ӧ�Ļ�ѧ����ʽΪ
CaH2+2H2O�TCa��OH��2+2H2��
CaH2+2H2O�TCa��OH��2+2H2��
����������1������̿���������ԣ���������ζ��ɫ�أ�
��2������ȼ�ղ�����ˮ����������ȼ�ϣ�д����Ӧ�Ļ�ѧ����ʽ���ɣ�
��3����հ�װ�dz�ȡ���еĿ�����ʹʳƷ���������������������������е��������ϸ����Ҳ�������к������ö���Ϣ������
��4�����ݷ��ӵĻ������ʽ��з������
��5���⻯�ƣ�CaH2����ˮ��Ӧ�����������ƺ�������д����Ӧ�Ļ�ѧ����ʽ���ɣ�
��2������ȼ�ղ�����ˮ����������ȼ�ϣ�д����Ӧ�Ļ�ѧ����ʽ���ɣ�
��3����հ�װ�dz�ȡ���еĿ�����ʹʳƷ���������������������������е��������ϸ����Ҳ�������к������ö���Ϣ������
��4�����ݷ��ӵĻ������ʽ��з������
��5���⻯�ƣ�CaH2����ˮ��Ӧ�����������ƺ�������д����Ӧ�Ļ�ѧ����ʽ���ɣ�
����⣺��1������̿���������ԣ���������ζ��ɫ�أ����³�����ԷŸ�����̿��ȥ����ζ�����л���̿���������ã�
��2������ȼ�ղ�����ˮ����������ȼ�ϣ���Ӧ�Ļ�ѧ����ʽΪ2H2+O2
2H2O��
��3��ʳƷ���ʳ�������հ�װ����䱣��������հ�װ��Ŀ����ʹʳƷ������������������ �����ֹʳƷ����������
��4�����ܷ����õķ������ƽԭ������ԭʱ����װ����������Ϊ��ԭ��������ѹ�ϵͣ����ڵ�������Ӽ�����
��5���⻯�ƣ�CaH2����ˮ��Ӧ�����������ƺ���������Ӧ�Ļ�ѧ����ʽΪCaH2+2H2O�TCa��OH��2+2H2����
�ʴ�Ϊ����1����������2��ʹʳƷ������������������ �����ֹʳƷ������������3��2H2+O2
2H2O����4�����ڵ�������Ӽ�����5��CaH2+2H2O�TCa��OH��2+2H2����
��2������ȼ�ղ�����ˮ����������ȼ�ϣ���Ӧ�Ļ�ѧ����ʽΪ2H2+O2
| ||
��3��ʳƷ���ʳ�������հ�װ����䱣��������հ�װ��Ŀ����ʹʳƷ������������������ �����ֹʳƷ����������
��4�����ܷ����õķ������ƽԭ������ԭʱ����װ����������Ϊ��ԭ��������ѹ�ϵͣ����ڵ�������Ӽ�����
��5���⻯�ƣ�CaH2����ˮ��Ӧ�����������ƺ���������Ӧ�Ļ�ѧ����ʽΪCaH2+2H2O�TCa��OH��2+2H2����
�ʴ�Ϊ����1����������2��ʹʳƷ������������������ �����ֹʳƷ������������3��2H2+O2
| ||
��������������������ص�֪ʶ���п�������ȵ�֮һ�������漰֪ʶ��϶࣬���������ѧ֪ʶ����ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
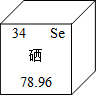 ��2013?��ɽ��һģ����Ԫ�ر���Ϊ������������������Ԫ�����ڱ��е���Ϣ��ͼ��ʾ����ͼ����Ϣ���Ͳ���ȷ���ǣ�������
��2013?��ɽ��һģ����Ԫ�ر���Ϊ������������������Ԫ�����ڱ��е���Ϣ��ͼ��ʾ����ͼ����Ϣ���Ͳ���ȷ���ǣ�������