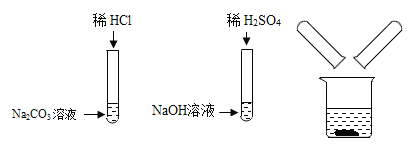
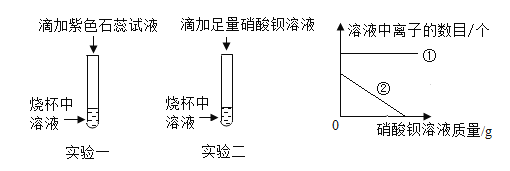
��Ŀ����
��Ԫ�����˱���ij���Ԫ�أ�������ÿ��ѪҺ����88��108 mg���ְ����в���ⶨijѪ���ĺ���������ȡѪ��10 mL����Ѫ���еĸ�Ԫ��ȫ��ת����CaC2O4�У�����ϡ���ᴦ��CaC2O4����Ӧ�Ļ�ѧ����ʽΪCaC2O4��H2SO4=CaSO4��H2C2O4���۲��������Ӧ������H2C2O4������Ϊ2.25 mg�����㲢�ж�(Ҫ��д���������)��
(1)ÿ10 mL��Ѫ����ת�����ɵ�CaC2O4������
(2)ÿ����Ѫ���и�Ԫ�ص����������жϸ�Ѫ���������Ƿ�������
��ϰ��ϵ�д�
�����Ŀ
��һ�������£��ס��ҡ������������������ܱ������з���ij����Ӧ����÷�Ӧǰ������ʵ��������±���
���� | �� | �� | �� | �� |
��Ӧǰ����/g | 20 | 30 | 20 | 15 |
��Ӧ������/g | 0 | x | y | 10 |
��������������ǣ�������
A.�μӷ�Ӧ�ļ��붡��������Ϊ4��1 B.x+y=75
C.y��20ʱ���÷�Ӧһ���ǻ��Ϸ�Ӧ D.x��ȡֵ��Χ��0��x��30


 ��
�� �ֱ��ʾ��ͬԪ�ص�ԭ�ӣ���ͼ�б�ʾ��������
�ֱ��ʾ��ͬԪ�ص�ԭ�ӣ���ͼ�б�ʾ��������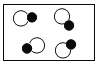 B.
B.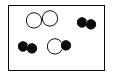 C.
C.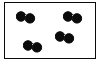 D.
D.