��Ŀ����
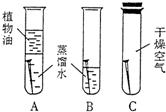 29����ͼ��С��ͬѧ������ʴ������̽��ʾ��ͼ��
29����ͼ��С��ͬѧ������ʴ������̽��ʾ��ͼ���پ���һ��ʱ������۲쵽����������Щ��
A��C�Թ��е���������û�б仯��B�Թ��е��������⣬�ڿ�����ˮ��ĽӴ��ط���������Ƚ϶�
����A�Թ��з�ֲ���͵�������ʲô��
��������������
����ͨ����������ʴ������̽�������õ�ʲô���ۣ�
���ڳ�ʪ�Ŀ�������������
��������������������ʩ��
��һ��
��������Ʒ����Ľྻ�����
�ڶ���
������Ʒ�ı���Ϳ��һ�㱣��Ĥ����Ϳһ������
��������������������Ǹ�����������ˮͬʱ�Ӵ���������Һ��������Һ������Һ�ܴٽ��������⣮
��ֹ��������ķ����У��ڽ�������Ϳһ������ڽ��������һ������ȣ�
��ֹ��������ķ����У��ڽ�������Ϳһ������ڽ��������һ������ȣ�
����⣺��A������û���������Ӵ���C��û����ˮ�Ӵ���A��C�Թ��е�����û�����⣮B�Թ��е�������������ˮ��ֽӴ����������⣮���A��C�Թ��е���������û�б仯��B�Թ��е��������⣬�ڿ�����ˮ��ĽӴ��ط���������Ƚ϶࣮
��A�Թ��з�ֲ���͵������Ǹ�������������������������ã�
��ͨ����������ʴ������̽����֪�����ڳ�ʪ�Ŀ������������⣮������ڳ�ʪ�Ŀ������������⣮
�ܷ�ֹ��������ķ����У���������Ʒ����Ľྻ����������Ʒ�ı���Ϳ��һ�㱣��Ĥ����Ϳһ�����ᣮ
��A�Թ��з�ֲ���͵������Ǹ�������������������������ã�
��ͨ����������ʴ������̽����֪�����ڳ�ʪ�Ŀ������������⣮������ڳ�ʪ�Ŀ������������⣮
�ܷ�ֹ��������ķ����У���������Ʒ����Ľྻ����������Ʒ�ı���Ϳ��һ�㱣��Ĥ����Ϳһ�����ᣮ
�����������Ҫ��������������������ֻ������������������������ҳ���ֹ������ķ�����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��11�֣������������ճ������г��ø�����ϴ����ѡ���ʵ�����Ʒ���Եõ����õ���ϴЧ����
| ���� | ϴ���� | ����� | ¯������ | ���ձ�ը�� | Ư�� |
| ��Ʒ��ʽ |  |  |  |  |  |
| ��Ч�ɷ� ���� | ��ϴ���� | ���� | �������� | ��̼���� | ���� |
��2���������ʿ���ʹ�ý������ϴ���� ����ĸ��ţ���
a������ b������ c��ˮ������Ҫ�ɷ�Ϊ̼��ƺ�������þ��
��3��ȡ����һ������¯���������μӼ��η�̪��Һ����Һ�� ɫ�������������¯��������ϣ����Է�����ͼ��ʾ�Ļ�ѧ��Ӧ��ͼ��a���Ļ�ѧʽΪ ��

��4�������ձ�ը�Ρ�����ˮ�������Na2CO3��H2O2������ը������ˮ���ټ��������Ľ���飬�����Ļ�ѧ��Ӧ����ʽΪ ��
��5����ѧС�鷢��һ����װ�����Ư�ۣ�ͬѧ�Ƕ�Ư����Ư�������Ƿ�ʧЧ���������ʡ�������Ч�ɷ���ȫ��ʧʱ����Ư�۾���ȫʧЧ��������ʧʱ����Ϊ����ʧЧ����
I���������ϣ�
��Ư�۵���Ҫ�ɷ���Ca(ClO)2��CaCl2��Ca(OH)2������Ч�ɷ���Ca(ClO)2��
��Ca(ClO)2������ˮ��Ư��ԭ���ǣ����ڿ����з�����Ӧ�� Ca(ClO)2+H2O+CO2=CaCO3��+2HClO��
��HClO���ȶ����ֽ�����HCl��һ�ֳ����ĵ������塣
�� CaCl2��ˮ��Һ�����ԣ�HClO��ˮ��Һ�����ԡ�
�� HClO�ܿ�ʹ��ɫ���ʣ��磺Ʒ����Һ����ɫ��

II���������ۣ�
��С��ͬѧ���������ó���HClO�ֽ�ʱ������HCl�⣬���ɵ���һ�ֳ��������� ��
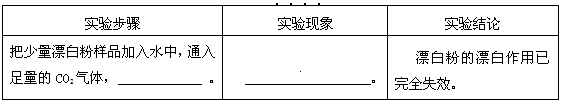
III��ʵ��̽���� �±���̽��ijƯ���Ƿ���ȫʧЧ��ʵ�飬����ݱ��н��ۣ�������ա�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ������Ư����Ʒ����ˮ�У�ͨ��������CO2���壬 �� | �� | Ư�۵�Ư����������ȫʧЧ�� |
����һ��CaCl2��CaCO3�� ������� ��
С��ͬѧ��Ϊ�����еijɷ�CaCO3����Ư�۵���Ч�ɷ��ڿ����з�����Ӧ�����⣬����������Դ�������û�ѧ����ʽ��ʾ ��
С��ͬѧ����ʵ��֤��ʧЧ���Ư�۵ijɷַ��ϲ���һ����������������С��ͬѧ��ʵ�鷽���ǣ� ��





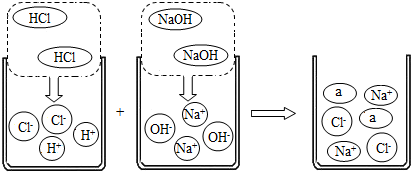
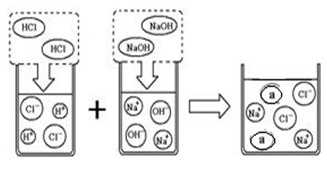
 С��ͬѧ�Ա�ǩ��ֻ�ܿ�����SO4����ijƿ��ɫ��Һ������ͼ���ijɷֽ�������̽�������������ʿ����ǣ�
С��ͬѧ�Ա�ǩ��ֻ�ܿ�����SO4����ijƿ��ɫ��Һ������ͼ���ijɷֽ�������̽�������������ʿ����ǣ� С��ͬѧ�Ա�ǩ��ֻ�ܿ�����SO4����ijƿ��ɫ��Һ������ͼ���ijɷֽ�������̽�������������ʿ����ǣ�
С��ͬѧ�Ա�ǩ��ֻ�ܿ�����SO4����ijƿ��ɫ��Һ������ͼ���ijɷֽ�������̽�������������ʿ����ǣ�