��Ŀ����
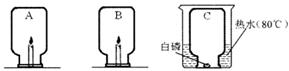
ijͬѧ��ѧϰ������������������������IJⶨ�������Լ������ͼ��ʾ��װ�ã�����������������ĺ�������ͬѧ��ʵ�鲽�����£�

�Ž�ͼ�еļ���ƿ��Ϊ5�ȷݣ������ñ�ǣ�
�ƽ������ϵ�ֹˮ�мн����ھƾ����ϵ�ȼ���ף�����������ƿ�ڣ�������Ƥ����
�dz�ַ�Ӧ����ȴ�����£���ֹˮ�С�
��ش��������⣺
�ٸ�ʵ���к������Թ�����Ŀ����_______ ��
�ڲ�����д�ֹˮ�к�۲쵽��������_______________ __________ _____���ɴ˿ɵó��������������������ԼΪ____ _______��
�۸�ʵ������۳�����_______����ѡ����ס�������ˮ���仯ѧ����________������á������á����Ľ��ۡ�
��ʵ��������������ٵ��������С���������ⶨ��1/5����װ�úͲ�����ʧ���������ԭ������� ����дһ�㼴�ɣ�

�Ž�ͼ�еļ���ƿ��Ϊ5�ȷݣ������ñ�ǣ�
�ƽ������ϵ�ֹˮ�мн����ھƾ����ϵ�ȼ���ף�����������ƿ�ڣ�������Ƥ����
�dz�ַ�Ӧ����ȴ�����£���ֹˮ�С�
��ش��������⣺
�ٸ�ʵ���к������Թ�����Ŀ����_______ ��
�ڲ�����д�ֹˮ�к�۲쵽��������_______________ __________ _____���ɴ˿ɵó��������������������ԼΪ____ _______��
�۸�ʵ������۳�����_______����ѡ����ס�������ˮ���仯ѧ����________������á������á����Ľ��ۡ�
��ʵ��������������ٵ��������С���������ⶨ��1/5����װ�úͲ�����ʧ���������ԭ������� ����дһ�㼴�ɣ�
�� ��ȫ�����꼯��ƿ�ڵ�������2�֣���ˮ������ƿ�У��ӽ�1/5�̶��ߴ���2�֣�21%��1�֣�
�� �ѣ�1�֣�����ѡ����ס����������ã�1�֣�������á������á���:
�ܺ��������㣨��δ��ȫ��ȴ�����µȣ�
�� �ѣ�1�֣�����ѡ����ס����������ã�1�֣�������á������á���:
�ܺ��������㣨��δ��ȫ��ȴ�����µȣ�
�������������ʵ���к������Թ�������ȷ��������������ȫ��Ӧ���Ծ��ⶨ������������ڳ�ַ�Ӧ����ȴ�����£���ֹˮ�У�ƿ������ļ��ٶ�ʹѹǿ��С���ձ���ˮ����ƿ�в���������ռ�������������ˮ�����Ϊԭ���������������ƿ��ʣ��������ҪΪ���������е�ˮ���ֻռƿ�ݻ���
 �����ж�ʣ��ĵ�����������ˮ������Ҳ�����뵪��������Ӧ�����жϵ�����ѧ���ʲ����ã�����������������������ȫ����������ȼ�պ�δ��ȫ��ȴ��ʣ�����������͵ȣ����������������ٵ����С��
�����ж�ʣ��ĵ�����������ˮ������Ҳ�����뵪��������Ӧ�����жϵ�����ѧ���ʲ����ã�����������������������ȫ����������ȼ�պ�δ��ȫ��ȴ��ʣ�����������͵ȣ����������������ٵ����С��

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����Ŀ