��Ŀ����
 ��2011?��Զ������������ܽ�ȱ����ܽ�����ش��������⣺
��2011?��Զ������������ܽ�ȱ����ܽ�����ش��������⣺| ����\�ܽ��\g�¶�\��C | 0 | 20 | 40 | 60 | 80 |
| KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 |
| NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
| Ca��OH��2 | 0.173 | 0.165 | 0.121 | 0.116 | 0.094 |
NaCl����ʳ�Σ�
NaCl����ʳ�Σ�
���ܽ�����ߣ���2��40��ʱ���Ȼ��Ƶ��ܽ��
�� ����
�� ����
�����ڡ�С�ڻ���ڣ�����ص��ܽ�ȣ���3��������л����������Ȼ��ƣ���Ҫ�õ�����������صķ�����
���أ��ᾧ
���أ��ᾧ
����4����ʹ����صIJ�������Һת��Ϊ������Һ�����Բ�ȡ�ķ���֮һ��
��KNO3�����壩��������ˮ�ݣ����¶ȣ�
��KNO3�����壩��������ˮ�ݣ����¶ȣ�
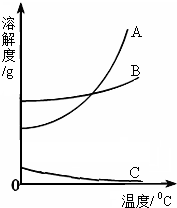
����5����ͼ��ʾ���ձ�A���DZ��͵�����������Һ�����ձ�B�м�����ʯ�Һ��ձ�A�б���ǣ����ܵ�ԭ����
B
B
������ţ��� A����Ӧ����ˮ��������������
A����Ӧ����ˮ��������������B����Ӧ���ȣ��¶����ߣ����������ܽ�Ƚ���
C����ʯ����ˮ��Ӧ���ɵ��������Ʋ����ܽ⣮
�����������ܽ�ȱ����ܽ�����߿�֪����1�����֪B���������ʵ��ܽ�����ߣ�
��2�����ܽ�ȱ����е����ݿ�֪��40��ʱ���Ȼ��Ƶ��ܽ��������ص��ܽ�ȵĴ�С��ϵ��
��3�������ܽ�����߿�֪��������л����������Ȼ��ƣ���Ҫ�õ�����������صķ�����
��4����Ϊ����ص��ܽ�����¶ȵ�Ӱ��ϴʿ�֪��ʹ����صIJ�������Һת��Ϊ������Һ�����Բ�ȡ�ķ�����
��5����ʯ������ˮ���ȣ����������Ƶ��ܽ�����¶ȵ����߶����ͣ��ձ�A�б���ǣ����ܵ�ԭ��
��2�����ܽ�ȱ����е����ݿ�֪��40��ʱ���Ȼ��Ƶ��ܽ��������ص��ܽ�ȵĴ�С��ϵ��
��3�������ܽ�����߿�֪��������л����������Ȼ��ƣ���Ҫ�õ�����������صķ�����
��4����Ϊ����ص��ܽ�����¶ȵ�Ӱ��ϴʿ�֪��ʹ����صIJ�������Һת��Ϊ������Һ�����Բ�ȡ�ķ�����
��5����ʯ������ˮ���ȣ����������Ƶ��ܽ�����¶ȵ����߶����ͣ��ձ�A�б���ǣ����ܵ�ԭ��
����⣺��1�������ܽ�ȱ����ܽ�����߿�֪��B��NaCl����ʳ�Σ����ܽ�����ߣ�
��2��
���ܽ�ȱ����е����ݿ�֪��40��ʱ���Ȼ��Ƶ��ܽ��С������ص��ܽ�ȣ�
��3�������ܽ�����߿�֪��������л����������Ȼ��ƣ���Ҫ�õ�����������صķ����ǣ��أ��ᾧ��
��4����Ϊ����ص��ܽ�����¶ȵ�Ӱ��ϴʿ�֪��ʹ����صIJ�������Һת��Ϊ������Һ�����Բ�ȡ�ķ���֮һ�Ǽ�KNO3�����壩��������ˮ�ݣ����¶ȣ���
��5����ͼ��ʾ���ձ�A���DZ��͵�����������Һ�����������Ƶ��ܽ�����¶ȵ����߶����ͣ������ձ�B�м�����ʯ�Һ���ʯ������ˮ���ȣ���Һ���¶����ߣ��ձ�A�б���ǣ����ܵ�ԭ���ǣ���Ӧ���ȣ��¶����ߣ����������ܽ�Ƚ��� ����ѡC��
����ѡC��
�ʴ�Ϊ����1��NaCl����ʳ�Σ�����2��С�� ������
��3�����أ��ᾧ����4����KNO3�����壩��������ˮ�ݣ����¶ȣ�����5��B��

��2��
| ����\�ܽ��\g�¶�\��C | 0 | 20 | 40 | 60 | 80 |
| KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 |
| NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
| Ca��OH��2 | 0.173 | 0.165 | 0.121 | 0.116 | 0.094 |
��3�������ܽ�����߿�֪��������л����������Ȼ��ƣ���Ҫ�õ�����������صķ����ǣ��أ��ᾧ��
��4����Ϊ����ص��ܽ�����¶ȵ�Ӱ��ϴʿ�֪��ʹ����صIJ�������Һת��Ϊ������Һ�����Բ�ȡ�ķ���֮һ�Ǽ�KNO3�����壩��������ˮ�ݣ����¶ȣ���
��5����ͼ��ʾ���ձ�A���DZ��͵�����������Һ�����������Ƶ��ܽ�����¶ȵ����߶����ͣ������ձ�B�м�����ʯ�Һ���ʯ������ˮ���ȣ���Һ���¶����ߣ��ձ�A�б���ǣ����ܵ�ԭ���ǣ���Ӧ���ȣ��¶����ߣ����������ܽ�Ƚ���
 ����ѡC��
����ѡC���ʴ�Ϊ����1��NaCl����ʳ�Σ�����2��С�� ������
��3�����أ��ᾧ����4����KNO3�����壩��������ˮ�ݣ����¶ȣ�����5��B��
�����������ܽ�������ܶ����ر�ʾ���ܽ�ȱ仯�Ĺ��ɣ��˽ⱥ����Һ�벻������Һ���ת�䷽����

��ϰ��ϵ�д�
�����Ŀ