��Ŀ����
����Ŀ�����գ��ҹ�����������ʵ�鷢���µĵ����������������ڷ����д�һ������ת�����һ�����ͣ�ͨ����Ϊ�������⽫Ϊ�ƽ������еġ������ʡ�֮�������µĽ�Կ�ס���ش�
����Ϣ�ṩ������1����ѧ�������������п��ܴ�����ȫ�ɷ����ӹ��ɵ����ʼ��������ʡ��� �����ӡ������ӵȶ��Ƿ����ӣ����Ǹ�ͨ����˵�ĵ��ӡ�������Ƚϣ�������ȵ������෴�������뷴������������������������ͷų��������������Դ�о�������ǰ���ɹۡ�
��2��������ƻô�Ƭ��2012������������̫���ͻȻ�Ӿ磬�ͷų����������ӣ��غ˱���Щ���Ӽ��Ȳ��ۻ��������˾��ҵĵ���ͻ�ɽ�����������������������֮�֡�
��3���ƻ�Ƭ���Ǽ��Ժ�����Ա���÷����������Ǽʷɴ�ȼ�Ͻ���̫��֮�á�����ʹ��ħ�����������ʱ�������������������Դ��
��Ӧ����չ����1���������������Ϣ�����Ʋ⣬����Ŀ�еķ���ԭ�ӽṹ��������_______��
A��һ��������ɵ�������һ��������ɵĵ��ӹ���
B��һ��������ɵ�������һ��������ɵĵ��ӹ���
C��һ��������ɵ�������һ��������ɵĵ��ӹ���
D��һ��������ɵ�������һ��������ɵĵ��ӹ���
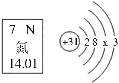
��2������Ϊ����ԭ�ӵĽṹʾ��ͼ�ɱ�ʾΪ![]() ,��ͼ�д��������ӵķ��ż�������ʾ�����壺+1��ʾ_________�� ��1��ʾ_______��
,��ͼ�д��������ӵķ��ż�������ʾ�����壺+1��ʾ_________�� ��1��ʾ_______��
��3���������뷴���ӡ������뷴��������ײ���ͻ�����������____���Ƿ�ѧ�仯����˵�����������_____��
���𰸡�B 1�������� ����ԭ�Ӻ�������ֻ��1�������ӣ���1�������ӣ� ���� ��ѧ�仯��ʵ���Ƿ������ѳ�ԭ�ӣ���ԭ����������µķ��ӡ�
��������
��1�������Ͽ�֪���������Ǹ�ͨ����˵���ӱȽϣ������еĵ��ӡ����ӣ�����ͨ�����е����Ӻ͵��ӣ�������ȵ������෴����ԭ�������Ӵ����磬����ԭ�������Ӵ����磬��ԭ���е��Ӵ����磬����ԭ���У����Ӵ����磬Bѡ��������⣬��ѡ��B
��2���������е���Ϣ:�����ӵ��ص���������������������Ӧ��������ͬ,����ɡ��žص���֮�෴,����֪����ԭ�ӵĽṹʾ��ͼ�ɱ�ʾΪ![]() ;��һ��������ɵ�������һ��������ɵĵ��ӹ��ɣ������ķ��ż�������ʾ������ֱ���: +1��ʾ1��������;-1��ʾ����ԭ�Ӻ�������ֻ��1��������(��1��������)
;��һ��������ɵ�������һ��������ɵĵ��ӹ��ɣ������ķ��ż�������ʾ������ֱ���: +1��ʾ1��������;-1��ʾ����ԭ�Ӻ�������ֻ��1��������(��1��������)

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�