��Ŀ����
��2007?���Ƹ�ģ�⣩ij��һѧϰС���ԡ������г��ϼ��ֲ��Ʊ�����Ʒ�ĵ����о���Ϊ���������̽��ѧϰ�������һ����������������̽�����̣�[�Ŀ��]
��1���˽��Ԫ�������彡���Ĺ�ϵ��
��2���˽�Ʋ�Ʒ�ĺ��������۸������Ƿ�ϸ�
��3��������ȡ��Ϣ��������Ϣ���������⡢������⡢����̽��������
[�����ռ�����]
��1��ͨ���г����飬���������ָƲ�Ʒ���й���Ϣ���£�
| Ʒ�� | �ƶ���-D | ��˼��-D | ������ | ������� |
| ��Ҫ�������� | ̼��� | ̼��� | ���������ϸ� | ������� |
| ��Ԫ�غ�����mg/Ƭ�� | 600 | 500 | 250 | 168 |
| ������Ƭ��/ƿ���У� | 30 | 20 | 30 | 30 |
| �۸�Ԫ��/ƿ���У� | 27.00 | 23.10 | 30.00 | 25.20 |
������������ÿ���������ӦΪ1 300 mg����ʵ����Ŀǰ�ҹ���ѧ��ÿ�ո��������ľ�ֵΪ518 mg��Ӫ��ѧ�ṫ���ľ���ÿ�ո�������Ϊ800 mg��
[�����]
��1���Ʊ�����Ʒ�еĸ�Ԫ����______��ʽ���ڣ� A������B��������
��2������ĸ�Ԫ����Ҫ������______�У�
��3����������������Ϣ������������ÿ�ո���Ҫ����ʵ�����������㣬Ӧע��______��
[����������]
��1�����ϱ������Ƚϣ����ָƲ�Ʒ�У���������ͬ�����ĸ�Ԫ��ʱ�۸���������______��
A���ƶ���-D B����˼��-D C��������D���������
��2���ڽ��������У�С��ͬѧ���һ���۵㣬���ƻ�����ʳ��������ʡǮ���ܲ��ƣ������������Բ��Ƶ���______��
A����ʳ����������ʯ��B��ʳ��һЩϺƤC�����Ź���D������ͷʱ�Ŵ���
��3��С����Ϊʳ�ÿ�˼��-D��̼��ƽ���θ���ܷ�����ѧ��Ӧ���仯ѧ����ʽ�ǣ�______��
[��������̽��]
�ɸƶ���-D˵����֪ÿƬ�ƶ���-D�к�̼���1.5 g������֪���ƶ���-D����������̼���Σ�����ϸ����ϡ������ȫ��Ӧ�����������У�Сǿͬѧ���һ�����⣬�����г�����Щ�������ӻ��Լ����棬����ĸƶ���-D��֪�Ƿ�ϸ���ͨ��ʵ�ʶ���������Сǿ��������·���������ʵ�飺ȡ50Ƭ�ƶ���-D��ϸ��������ƿ������ͼ�������������������ַ�Ӧ���������ͨ������������Һ����������ȫ���������������գ�����ϵͳ�ڿ�����CO2��Ӱ�죩�����γ���ʢ����������Һ��ϴ��ƿ�ɼ������Ʒ�Ƿ�ϸ��Իش��������⣺
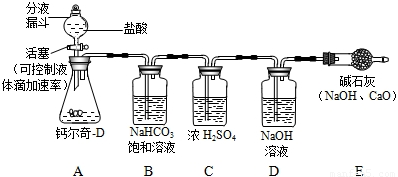
��1��Сǿ��B��NaHCO3������Һ��ȥCO2�л��е�����HCl���壬B�е�NaHCO3��Һ�ܷ�ʯ��ˮ������ܡ�������������______��
��2��С����Ϊ��Сǿ����Ʋ��ܴﵽ������Ʒ�Ƿ�ϸ��Ŀ�ģ�Ӧ�ü��ԸĽ������õ���С������ͬѧ����ͬ������ΪС�����ֵ�������______���Ľ��ķ�����______��
��3���øĽ����װ�ú�ҩƷ����ʵ�飬����ʢ����������Һ��ϴ��ƿ������33 g����ͨ������˵��Сǿѡ�õĸƶ���-D�Ƿ�Ϊ�ϸ��Ʒ��
���𰸡�������[�о�����]��1�����������иƵĴ�����ʽ�ش�
��2�����������иƵĴ���λ�ûش�
��3�����������иƵ��������ܻش�
[����������]��1�����ݸ���ҩƷ����Ҫ�ɷ��к��и�Ԫ�ص���������۸�ȱȽϻش�
��2�����ݸ�Ԫ�ص���Ҫʳ����Դ�ش�
��3������θ��ijɷ�������̼��ƵĻ�ѧ���ʻش�
[��������̽��]��1�����ݶ�����̼��NaHCO3������Һ�Ļ�ѧ���ʻش�
��2������̼�����ƺ����ᷴӦ�����˶�����̼������ش�
��3������ʢ����������Һ��ϴ��ƿ������33 g���ж����ɵĶ�����̼���������ٸ��ݶ�����̼���������̼��Ƶ�����������ж��Ƿ�ϸ�
����⣺[�о�����]��1���Ʊ�����Ʒ�еĸ�Ԫ�ض����Ի��������ʽ���ڣ�
��2���������ڵĸ�Ԫ����Ҫ�����ڹǸ�������У�
��3���������ȱ�ƣ�����������Ỽ���Ͳ��ͷ�������������Ҫע�ⲹ�ƣ�
[����������]��1��600mg/Ƭ×30Ƭ÷27.00Ԫ=666.67 mg/Ԫ��500mg/Ƭ×20Ƭ÷23.10Ԫ=432.9 mg/Ԫ��250mg/Ƭ×30Ƭ÷30.00Ԫ=250 mg/Ԫ��168mg/Ƭ×30Ƭ÷25.20Ԫ=200 mg/Ԫ������ѡA��
��2��A����ʳ����������ʯ�ң���ʯ�һḯʴ�˵���������B��ʳ��һЩϺƤ���ԣ�ϺƤ�и����ƣ�C�����Ź������ԣ���ͷ���к���Ԫ�ضࣻD������ͷʱ�Ŵ�����ԣ�������̼���ƣ�������Ԫ�أ�
��3��θ�����Ҫ�ɷ������ᣬ����̼��Ʒ�Ӧ����������̼��ˮ�Ͷ�����̼������ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
[��������̽��]��1��B�е�NaHCO3��Һ���ܻ���ʯ��ˮ����Ϊʯ��ˮ����Ҫ�ɷ����������ƣ��ܺͶ�����̼Ҳ������Ӧ��
��2��NaHCO3������Һ����HClʱ��Ҫ��Ӧ����CO2������ʢNaOH��Һ��ϴ��ƿ���ش�����Ʒ����CO2����������������Ʒ�Ƿ�ϸ����ỻ��ϡ���ᣬ�Ͳ������Ȼ�������ӷ��������ٽ�NaHCO3������Һ��ϴ��ƿȥ���Ϳ����ˣ�
��3����ƶ���-D��̼��Ƶ�����ΪX
����ʢ����������Һ��ϴ��ƿ������33 g�������ɵĶ�����̼������33 g��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 33g
100÷44=X÷33g X=75g
75g÷50Ƭ=1.5g/Ƭ ��˵����һ�£�Ϊ�ϸ��Ʒ��
�ʴ�Ϊ��[�о�����]��1��B��2�����������ݣ�3�����Ƶ�
[����������]��1��A��2��BC��3��CaCO3+2HCl=CaCl2+H2O+CO2��
[��������̽��]��1������Ϊʯ��ˮҲ�����ն�����̼����CO2����
��2��NaHCO3������Һ����HClʱ��Ҫ��Ӧ����CO2������ʢNaOH��Һ��ϴ��ƿ���ش�����Ʒ����CO2����������������Ʒ�Ƿ�ϸ����ỻ��ϡ���ᣬ��NaHCO3������Һ��ϴ��ƿȥ����
��3���⣺��ƶ���-D��̼��Ƶ�����ΪX
����ʢ����������Һ��ϴ��ƿ������33 g�������ɵĶ�����̼������33 g��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 33g
100÷44=X÷33g X=75g
75g÷50Ƭ=1.5g/Ƭ
��˵����һ�£�Ϊ�ϸ��Ʒ��
��������ѧ��Դ�����������Ҳ�����������������������������������йصĻ�ѧ֪ʶ��������ȵ�֮һ��
��2�����������иƵĴ���λ�ûش�
��3�����������иƵ��������ܻش�
[����������]��1�����ݸ���ҩƷ����Ҫ�ɷ��к��и�Ԫ�ص���������۸�ȱȽϻش�
��2�����ݸ�Ԫ�ص���Ҫʳ����Դ�ش�
��3������θ��ijɷ�������̼��ƵĻ�ѧ���ʻش�
[��������̽��]��1�����ݶ�����̼��NaHCO3������Һ�Ļ�ѧ���ʻش�
��2������̼�����ƺ����ᷴӦ�����˶�����̼������ش�
��3������ʢ����������Һ��ϴ��ƿ������33 g���ж����ɵĶ�����̼���������ٸ��ݶ�����̼���������̼��Ƶ�����������ж��Ƿ�ϸ�
����⣺[�о�����]��1���Ʊ�����Ʒ�еĸ�Ԫ�ض����Ի��������ʽ���ڣ�
��2���������ڵĸ�Ԫ����Ҫ�����ڹǸ�������У�
��3���������ȱ�ƣ�����������Ỽ���Ͳ��ͷ�������������Ҫע�ⲹ�ƣ�
[����������]��1��600mg/Ƭ×30Ƭ÷27.00Ԫ=666.67 mg/Ԫ��500mg/Ƭ×20Ƭ÷23.10Ԫ=432.9 mg/Ԫ��250mg/Ƭ×30Ƭ÷30.00Ԫ=250 mg/Ԫ��168mg/Ƭ×30Ƭ÷25.20Ԫ=200 mg/Ԫ������ѡA��
��2��A����ʳ����������ʯ�ң���ʯ�һḯʴ�˵���������B��ʳ��һЩϺƤ���ԣ�ϺƤ�и����ƣ�C�����Ź������ԣ���ͷ���к���Ԫ�ضࣻD������ͷʱ�Ŵ�����ԣ�������̼���ƣ�������Ԫ�أ�
��3��θ�����Ҫ�ɷ������ᣬ����̼��Ʒ�Ӧ����������̼��ˮ�Ͷ�����̼������ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
[��������̽��]��1��B�е�NaHCO3��Һ���ܻ���ʯ��ˮ����Ϊʯ��ˮ����Ҫ�ɷ����������ƣ��ܺͶ�����̼Ҳ������Ӧ��
��2��NaHCO3������Һ����HClʱ��Ҫ��Ӧ����CO2������ʢNaOH��Һ��ϴ��ƿ���ش�����Ʒ����CO2����������������Ʒ�Ƿ�ϸ����ỻ��ϡ���ᣬ�Ͳ������Ȼ�������ӷ��������ٽ�NaHCO3������Һ��ϴ��ƿȥ���Ϳ����ˣ�
��3����ƶ���-D��̼��Ƶ�����ΪX
����ʢ����������Һ��ϴ��ƿ������33 g�������ɵĶ�����̼������33 g��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 33g
100÷44=X÷33g X=75g
75g÷50Ƭ=1.5g/Ƭ ��˵����һ�£�Ϊ�ϸ��Ʒ��
�ʴ�Ϊ��[�о�����]��1��B��2�����������ݣ�3�����Ƶ�
[����������]��1��A��2��BC��3��CaCO3+2HCl=CaCl2+H2O+CO2��
[��������̽��]��1������Ϊʯ��ˮҲ�����ն�����̼����CO2����
��2��NaHCO3������Һ����HClʱ��Ҫ��Ӧ����CO2������ʢNaOH��Һ��ϴ��ƿ���ش�����Ʒ����CO2����������������Ʒ�Ƿ�ϸ����ỻ��ϡ���ᣬ��NaHCO3������Һ��ϴ��ƿȥ����
��3���⣺��ƶ���-D��̼��Ƶ�����ΪX
����ʢ����������Һ��ϴ��ƿ������33 g�������ɵĶ�����̼������33 g��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 33g
100÷44=X÷33g X=75g
75g÷50Ƭ=1.5g/Ƭ
��˵����һ�£�Ϊ�ϸ��Ʒ��
��������ѧ��Դ�����������Ҳ�����������������������������������йصĻ�ѧ֪ʶ��������ȵ�֮һ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ