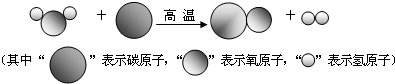
��Ŀ����
���ž��õķ�չ����Դ�뻷����Ϊ���������ע�����⣮��1����ʯȼ����Ҫ����ú��________��ʯ�ͣ�
��2����ʯȼ��ȼ�ն������������̼�����ǿ����к��������������壮��Ȼ�������Ķ�����̼����Ҫ;����________��
��3����̼������һ���Ե��ܺĺ�Ч�ܵ�Ϊ��Ҫ�������Խ��ٵ����������ŷŻ�ýϴ�������¾��÷�չģʽ�����з�����ʽ���ϡ���̼���á�������ǣ���������ţ�________��
��ˮ������ �ڷ������� ����Ȼ������ ��̫���ܷ��� ���������շ���
��4��������̼�ǵ�������ЧӦ����Ҫ���壬ͬʱ����Ҳ��һ�ֱ����̼����Դ��
��CO2��NH3�ϳ�����CO��NH2��2�ǹ̶�������CO2�ijɹ��������÷�Ӧ���ڸ��¸�ѹ�½��У�ͬʱ��ˮ���ɣ�д����Ӧ�Ļ�ѧ����ʽ________��
��5���������ʹ�÷���������Ҫ������������������ѧ�ҷ��ַ������������������·ֽ�õ�����ԭ�����ƻ������㣬���ƻ��������ѭ��ʾ��ͼ��ͼ��ʾ��
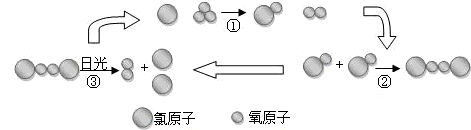
��ش������ϳ������ӱ��ƻ��Ĺ����У���ԭ�������������________��ʵ���һ��ɽ�����ͨ����ѹ�ŵ������ȡ���������ڷ�Ӧ����30%������ת��Ϊ�����������û����������������ķ��Ӹ�����Ϊ________��
�⣺��1������ʯȼ����ú��ʯ�ͺ���Ȼ�����ʴ�Ϊ����Ȼ��
��2����Ȼ�������Ķ�����̼����Ҫ;���ǣ���ɫֲ��Ĺ�����ã��ʴ�Ϊ���������
��3����ˮ������ �ڷ��������̫���ܷ��綼����ȼ�ջ�ʯȼ�ϣ��Ƚϻ������ʴ�Ϊ���٢ڢ�
��4��CO2��NH3��Ӧ��������CO��NH2��2��ˮ��Ȼ����ƽ���ɣ��ʴ�Ϊ��CO2+2NH3�TCO��NH2��2+H2O
��5������3O2=2O3���ڷ�Ӧ����30%������ת��Ϊ���������3��30%=10��ԭ����10�������ӣ���3���μ��˻�ѧ��Ӧ��������л�ʣ��7����������2���������ӣ���˻����������������ķ��Ӹ�����Ϊ7��2���ʴ�Ϊ������7��2
��������1���ɿα�֪ʶ�˽⻯ʯȼ����Ҫָʲô��
��2����Ȼ�������Ķ�����̼����Ҫ;����ֲ��Ĺ�����ã�
��3��������̼���õ���Ҫ���������ʵ�����⣻
��4�����������Ϣд����صĻ�ѧ����ʽ��ע����ƽ��
��5��������Ӧǰ�������ͻ�ѧ���ʲ��䣬Ȼ�������Ŀ����������Ϣ���ڷ�Ӧ����30%������ת��Ϊ�����������û����������������ķ��Ӹ����ȣ�
�����������������й�̼��ѭ�����⣬ͬʱ�����˴�������ѧ����ʽ����д�����������Լ��йصļ��㣬�ۺ��ԱȽ�ǿ���ر��ǻ�ѧ����ʽ����д���йصļ����DZ����һ���ѵ㣬Ҳ���״��㣬ϣ��ͬѧ��ץס��Ŀ��������Ϣ������������ۺϰ��գ�
��2����Ȼ�������Ķ�����̼����Ҫ;���ǣ���ɫֲ��Ĺ�����ã��ʴ�Ϊ���������
��3����ˮ������ �ڷ��������̫���ܷ��綼����ȼ�ջ�ʯȼ�ϣ��Ƚϻ������ʴ�Ϊ���٢ڢ�
��4��CO2��NH3��Ӧ��������CO��NH2��2��ˮ��Ȼ����ƽ���ɣ��ʴ�Ϊ��CO2+2NH3�TCO��NH2��2+H2O
��5������3O2=2O3���ڷ�Ӧ����30%������ת��Ϊ���������3��30%=10��ԭ����10�������ӣ���3���μ��˻�ѧ��Ӧ��������л�ʣ��7����������2���������ӣ���˻����������������ķ��Ӹ�����Ϊ7��2���ʴ�Ϊ������7��2
��������1���ɿα�֪ʶ�˽⻯ʯȼ����Ҫָʲô��
��2����Ȼ�������Ķ�����̼����Ҫ;����ֲ��Ĺ�����ã�
��3��������̼���õ���Ҫ���������ʵ�����⣻
��4�����������Ϣд����صĻ�ѧ����ʽ��ע����ƽ��
��5��������Ӧǰ�������ͻ�ѧ���ʲ��䣬Ȼ�������Ŀ����������Ϣ���ڷ�Ӧ����30%������ת��Ϊ�����������û����������������ķ��Ӹ����ȣ�
�����������������й�̼��ѭ�����⣬ͬʱ�����˴�������ѧ����ʽ����д�����������Լ��йصļ��㣬�ۺ��ԱȽ�ǿ���ر��ǻ�ѧ����ʽ����д���йصļ����DZ����һ���ѵ㣬Ҳ���״��㣬ϣ��ͬѧ��ץס��Ŀ��������Ϣ������������ۺϰ��գ�

��ϰ��ϵ�д�
�����Ŀ