��Ŀ����
̼�и��Ӵ�ļ��塣
��1��̼���ʾ��ж��ص����ʺ���;���������¿ո���д�������ƣ�
����Ȼ���ڵ���Ӳ������ ����Ǧ��о����Ҫ�ɷ�֮һ ��
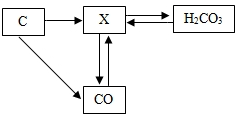
��2��̼�Ͳ���̼�Ļ������ת����ϵ����ͼ��ʾ��
������X�Ļ�ѧʽΪ ��
�������ʵķ����У�CO���� �������ţ�
| A���� | B���� | C���� | D�������� |
��3������л����Ǽ��飬��ȼ�յĻ�ѧ��Ӧ����ʽΪ ���������õ����ĺϳ��л��߷��Ӳ����� �����֡�
��4������̼���á��ĺ����ǽ��ܼ��š�����ͼ�пɿ�����

�ٵ��¿����ж�����̼���ӵ������� ����д��ţ���
��ͨ���������Ķ�����̼�Ļ�ѧ��Ӧ����ʽΪ��
��
������ЧӦ�ĺ���� ��дһ������
��10�֣���1���ٽ��ʯ ��ʯī ��2����CO2 ��D ��<
��3��CH4 + 2O2  CO2 + 2H2O ���ϡ��ϳ����ϳ���ά��
CO2 + 2H2O ���ϡ��ϳ����ϳ���ά��
��4���٢ڢ� CO2 + H2O ==H2CO3 �������ߵ��±����ۻ����������ɣ�
����

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
�����Ŀ
 ̼�и��Ӵ�ļ��壮
̼�и��Ӵ�ļ��壮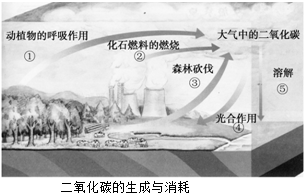


 ̼�и��Ӵ�ļ��壮
̼�и��Ӵ�ļ��壮