��Ŀ����
��ʳ����һֱ���������࣮1824�꣬�¹���ѧ��ά���״��˹��ϳ����أ���־�Ż�ѧ���ϵĵ�����֮����ȫ�����ձ�Ӧ�ã�ʹ��ʳ���������������[���ʳɷֳ�������]С��ͬѧ�ڼ����һ�����ʣ����ǩ��������ͼ��ʾ����
���������л����ǰ�ɫ���壬���������ϸ���ʯ��һ����ĥ����Ũ�ҵĴ̼�����ζ���ж����ʵ���Ҫ�ɷֵĻ�ѧʽ��д���ұ߱�ǩ�У����ɴ����뵽ʩ�øû���ʱӦע��

| �ƽ���� ��ѧʽ��___________ |
[��չ��Ǩ��]С�����뵽�ܷ��øû������������ƹ����ϼ����ư������������Ϸ��ְ�����һ���ܶȱȿ���С���Ҽ�������ˮ�����壬�ڳ���������HCl���壬������������̣����̵���Ҫ�ɷ������������廯���γɵ��Ȼ�淋Ĺ���С���������ż������Ȥ��С��������ȡ����İ�����Ȼ����������̽����HCl����ķ�Ӧ���������ۣ����������ʵ��װ�ã�

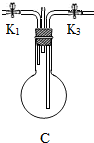
ע������װ�õ����������ã���ͼ1�е�B��Cװ�û������ƣ��д���ͬѧ����ʵ�������ѡ��Ͳ��䣩��Dװ�õ��Թ�����һ��ʪ��ĺ�ɫʯ����ֽ��Eװ�õ����������շ�Ӧ������ʣ�ఱ������ֹ���������������Ⱦ����ʯ�ҹ������������ƺ���ʯ�ҵĻ���
��1����д��Aװ���ڷ�Ӧ�Ļ�ѧ����ʽ��
��2���������С���̽����
| ʵ��Ŀ�� | ʵ����������� |
| ��ȡһ��ƿ����İ��� | �ٰ�ͼ1װ���������Լ�������ͼ1�����ڵ�Bװ��Ӧѡ��ͼ2�е�____������ţ�Bװ�õ�������____________________����ѡ����һװ�õ�ԭ���ǣ�________________________������Cװ�õ���ƿ�ڻ�������ֹˮ��K1��K3�ĵ��ܳ��������ʾ��ͼ�� �ڴ�ֹˮ��K1��K3���ر�ֹˮ��K2����ȼ�ƾ��Ƽ��ȣ�������__________����ʱ��˵��C��������������ֹͣ���ȣ����ر�ֹˮ��K1��K3��װ��E�и���ܵ�������____________________________ |
| ̽��������HCl����ķ�Ӧ | �۴�ֹˮ��K2��һ��ʱ����˿����д�������������һ�����⣬���ɹ۲쵽____________________��������һ�������Ҫԭ����______________�� |
������[���ʳɷֳ�������]
��ʯ����Ca��OH��2����������������Ʒ�Ӧ��������ơ�ˮ�Ͱ������ݴ�д�����ʵ���Ҫ�ɷֵĻ�ѧʽ�����ݻ�ѧ����ʽ�е��������ʵ����ʣ������ж�ʩ�øû���ʱӦע������⣻
[���ʳɷֵĽ�һ���ⶨ]
�������ᰱ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɰ������������г�����ʽ�����ɼ������Ʒ�У�NH4��2SO4��������Ȼ���������������ʽ���ɼ�������ʵĴ��Ⱥͣ�NH4��2SO4�е�Ԫ�صĺ�����
[��չ��Ǩ��]
��1����ʯ����Ca��OH��2����������������Ʒ�Ӧ��������ơ�ˮ�Ͱ������ݴ�д����ѧ����ʽ��
��2������ʵ�����Ҫ�������������ȷ���
��ʯ����Ca��OH��2����������������Ʒ�Ӧ��������ơ�ˮ�Ͱ������ݴ�д�����ʵ���Ҫ�ɷֵĻ�ѧʽ�����ݻ�ѧ����ʽ�е��������ʵ����ʣ������ж�ʩ�øû���ʱӦע������⣻
[���ʳɷֵĽ�һ���ⶨ]
�������ᰱ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɰ������������г�����ʽ�����ɼ������Ʒ�У�NH4��2SO4��������Ȼ���������������ʽ���ɼ�������ʵĴ��Ⱥͣ�NH4��2SO4�е�Ԫ�صĺ�����
[��չ��Ǩ��]
��1����ʯ����Ca��OH��2����������������Ʒ�Ӧ��������ơ�ˮ�Ͱ������ݴ�д����ѧ����ʽ��
��2������ʵ�����Ҫ�������������ȷ���
����⣺[���ʳɷֳ�������]
��ʯ����Ca��OH��2�������������ϸ���ʯ��һ����ĥ����Ũ�ҵĴ̼�����ζ������Ϊ��������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ����NH4��2SO4+Ca��OH��2
CaSO4��+2NH3��+2H2O��������Ũ�ҵĴ̼�����ζ���ʿ��ƶϻ��ʵ���Ҫ�ɷֵĻ�ѧʽΪ����NH4��2SO4����Ϊ��Ӧ����������Ƴ�������ʩ�øû���ʱӦע�ⲻ�ܺͼ������ʹ��ã�������ʧ��Ч��
[���ʳɷֵĽ�һ���ⶨ]
����Ʒ�У�NH4��2SO4������Ϊx��
��NH4��2SO4+Ca��OH��2
CaSO4��+2NH3��+2H2O
132 34
x 1.7g
132��34=x��1.7g
��֮�ã�x=6.6g��
���ʵĴ���Ϊ��
��100%=82.5%��
��NH4��2SO4�е�Ԫ�صĺ���Ϊ��
��100%��21.2%��
�����е�Ԫ�صĺ���Ϊ��82.5%��21.2%=17.5%��
�ʺ�����ӦΪ17.5%�����ʵĴ���ӦΪΪ82.5%��
[��չ��Ǩ��]
��1����������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ����NH4��2SO4+Ca��OH��2
CaSO4��+2NH3��+2H2O��
��2����ʵ�����������ͼ1�����ڵ�Bװ��Ӧѡ��ͼ2�еĢ٣�������������ˮ�����ΪŨ��������ˮ��ͬʱ���백����Ӧ���ʲ��â�װ�ã�
�ڵ�ȼ�ƾ��Ƽ���ʱ����D��ʯ����ֽ����ʱ��˵��C����������������Ϊ��ˮ�������ԣ���װ��E�и���ܵ������Ƿ�ֹˮ������
�۴�ֹˮ��K2һ��ʱ����˿��Կ����д���������һ�����⣬���ɿ����������ͣ���Ҫԭ�������巴Ӧ��IJ���Ϊ���壬�ڲ���ѹ��С��
�ʴ�Ϊ�����ܺͼ������ʹ��ã����������𰸾��ɣ���
[���ʳɷֵĽ�һ���ⶨ]
������ӦΪ17.5%�����ʵĴ���ӦΪΪ82.5%��
��1����NH4��2SO4+Ca��OH��2
CaSO4+2H2O+2NH3��
��ʯ����Ca��OH��2�������������ϸ���ʯ��һ����ĥ����Ũ�ҵĴ̼�����ζ������Ϊ��������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ����NH4��2SO4+Ca��OH��2
| ||
[���ʳɷֵĽ�һ���ⶨ]
����Ʒ�У�NH4��2SO4������Ϊx��
��NH4��2SO4+Ca��OH��2
| ||
132 34
x 1.7g
132��34=x��1.7g
��֮�ã�x=6.6g��
���ʵĴ���Ϊ��
| 6.6g |
| 8g |
��NH4��2SO4�е�Ԫ�صĺ���Ϊ��
| 14��2 |
| 132 |
�����е�Ԫ�صĺ���Ϊ��82.5%��21.2%=17.5%��
�ʺ�����ӦΪ17.5%�����ʵĴ���ӦΪΪ82.5%��
[��չ��Ǩ��]
��1����������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ����NH4��2SO4+Ca��OH��2
| ||
��2����ʵ�����������ͼ1�����ڵ�Bװ��Ӧѡ��ͼ2�еĢ٣�������������ˮ�����ΪŨ��������ˮ��ͬʱ���백����Ӧ���ʲ��â�װ�ã�
�ڵ�ȼ�ƾ��Ƽ���ʱ����D��ʯ����ֽ����ʱ��˵��C����������������Ϊ��ˮ�������ԣ���װ��E�и���ܵ������Ƿ�ֹˮ������
�۴�ֹˮ��K2һ��ʱ����˿��Կ����д���������һ�����⣬���ɿ����������ͣ���Ҫԭ�������巴Ӧ��IJ���Ϊ���壬�ڲ���ѹ��С��
�ʴ�Ϊ�����ܺͼ������ʹ��ã����������𰸾��ɣ���
| �ƽ���� ��ѧʽ����NH4��2SO4 |

[���ʳɷֵĽ�һ���ⶨ]
������ӦΪ17.5%�����ʵĴ���ӦΪΪ82.5%��
��1����NH4��2SO4+Ca��OH��2
| ||
| ʵ��Ŀ�� | ʵ����������� |
| �٢���ˮ���Ũ����ͬʱ���백����Ӧ ͼʾ���� ��D��ʯ����ֽ���� ��ֹˮ���� | |
| ���������ͣ����巴Ӧ��IJ���Ϊ���壬�ڲ���ѹ��С�� |
������������Ҫ�ǿ���ͬѧ�ǵ��ۺϷ�������������Ҫ��ͬѧ�Ǿ߱��й����ʵĻ���֪ʶ������Ҫ��ʵ������ľ����ͷ����������ѧʵ����������������ⲽ��϶࣬ѧ���ڽ���ʱ�����㹻�����ĺ�ϸ�ģ�������ȷ���

��ϰ��ϵ�д�
�����Ŀ

 ��ʳ����һֱ���������࣬1824�꣬�¹���ѧ��ά���״��˹��ϳ����أ���־�Ż�ѧ���ϵĵ�����֮�ʲ�ȫ�����ձ�Ӧ�ã�ʹ��ʳ���������������
��ʳ����һֱ���������࣬1824�꣬�¹���ѧ��ά���״��˹��ϳ����أ���־�Ż�ѧ���ϵĵ�����֮�ʲ�ȫ�����ձ�Ӧ�ã�ʹ��ʳ���������������