��Ŀ����
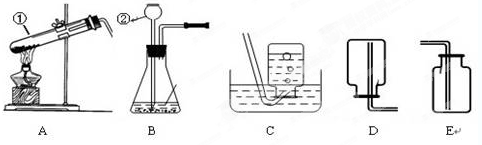
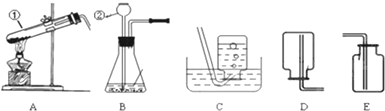
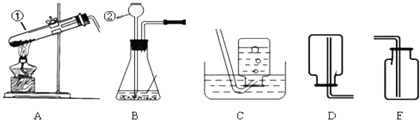
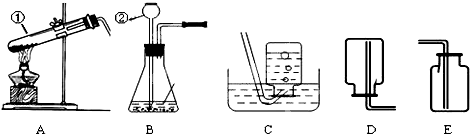
����ѧϰ����һ�����������������Ʊ�������������ͼʵ��װ��ͼ���ش����⣺
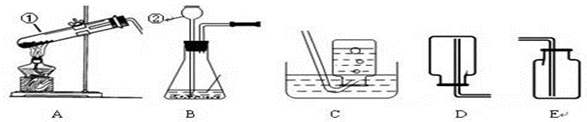
��1��д��������ŵ��������ƣ���______����______��
��2����ʵ�������ø��������ȡ�����Ļ�ѧ��Ӧԭ����______��
��3��ͼ��װ��A�����Դ�����ָ��______��������ʵ�������������ȡ��������ѡ��ķ���װ��Ϊ______����дװ�õ���ĸ���ţ���ͬ����Ӧѡ�õ��ռ�װ����______��
��4�������谷��ѧʽΪC3H6N6����һ�ֻ���ԭ�ϣ��������ԣ����㣺
��1�������谷��Է���������
��2�������谷��̼���⡢��Ԫ�ص������ȣ�
��3�������谷�е�Ԫ�ص�������������ȷ��0.1%����
��5��С���Ӽ�������һƿ���õ�ҽ�ù���������Һ����ͬѧ��һ��ⶨ�����Ǵ�ƿ��ȡ������Һ��51g�����������������̣����������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
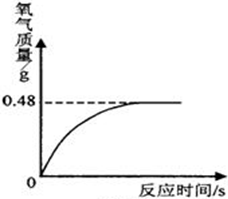
�����Һ������˫��ˮ��ʵ�������Ƕ��ٿˣ�
�⣺��1��Ҫ��Ϥ�������������ƺ����ã�
��2��д����ʽҪע��һд������ע���ĵȺţ��ø��������������ƽ�������ù۲취��ƽ����Ӧ���Ǹ�����أ�������������ء��������̺�������
��3�����������ȡ�����Թܿ�Ҫ��������б����ֹҩƷ�е�ˮ����������ˮ�ε������Թܵײ���ʹ�Թ�ը�ѣ����������Թ��ڲ���̫������������������ų�����Ӧװ�õ�ѡ���Ϊ���̹̼����͡���Һ�������ͣ�����غͶ������̶��ǹ�����Ҫ���ȣ�����װ��ѡ��A������������������ˮ����ˮ���ռ����ܶȱȿ������������ſ������ռ���
��4���������谷��Է�������=̼�����ԭ��������̼ԭ�Ӹ���+��Ԫ�����ԭ����������ԭ�Ӹ���+�������ԭ����������ԭ�Ӹ���=12��3+1��6+14��6�T126
��Ԫ������=Ԫ�ص����ԭ����������Ԫ�ص�ԭ�Ӹ���������̼Ԫ����������Ԫ����������Ԫ�������T12��3��1��6��14��6�T36��6��84�T6��1��14
�������谷�е�Ԫ�ص����������T ��100%�T66.7%
��100%�T66.7%
��5���⣺��ͼ���֪��������������Ϊ0.48g
��5 1g����������Һ�к��������������Ϊx��
2H2O2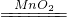 2H2O+O2��
2H2O+O2��
68 32
x 0.48 g
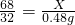 ���X=1.02g��
���X=1.02g��
�𣺸���Һ������˫��ˮ��ʵ������Ϊ1.02 g
�ʴ�Ϊ����1���Թܡ�����©��
��2��2KMnO4 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��
��3���Թܿ�δ��������б�������������Թ���̫����A��C��E
��4����126
��6��1��14
�������谷�е�Ԫ�ص���������66.7%
��1.02g
��������1��Ҫ��Ϥ�������������ƺ����ã���2�����ݷ���ʽ��д�����ǣ���3�����ݸ��������ȡ������ע������ͷ���װ�á��ռ�װ�õ�ѡȡ�������ǣ���4��������Է��������ļ��㡢Ԫ�������ļ��㡢Ԫ�����������ļ��㷽���ٽ��ͼ������Ϣ�ش�
������ͨ���ش���֪���˷���װ�á��ռ�װ�õ�ѡȡ���������������ȡ�����ķ�Ӧԭ������һ����������Է��������ļ��㡢Ԫ�������ļ��㡢Ԫ�����������ļ��㷽����
��2��д����ʽҪע��һд������ע���ĵȺţ��ø��������������ƽ�������ù۲취��ƽ����Ӧ���Ǹ�����أ�������������ء��������̺�������
��3�����������ȡ�����Թܿ�Ҫ��������б����ֹҩƷ�е�ˮ����������ˮ�ε������Թܵײ���ʹ�Թ�ը�ѣ����������Թ��ڲ���̫������������������ų�����Ӧװ�õ�ѡ���Ϊ���̹̼����͡���Һ�������ͣ�����غͶ������̶��ǹ�����Ҫ���ȣ�����װ��ѡ��A������������������ˮ����ˮ���ռ����ܶȱȿ������������ſ������ռ���
��4���������谷��Է�������=̼�����ԭ��������̼ԭ�Ӹ���+��Ԫ�����ԭ����������ԭ�Ӹ���+�������ԭ����������ԭ�Ӹ���=12��3+1��6+14��6�T126
��Ԫ������=Ԫ�ص����ԭ����������Ԫ�ص�ԭ�Ӹ���������̼Ԫ����������Ԫ����������Ԫ�������T12��3��1��6��14��6�T36��6��84�T6��1��14
�������谷�е�Ԫ�ص����������T
 ��100%�T66.7%
��100%�T66.7%��5���⣺��ͼ���֪��������������Ϊ0.48g
��5 1g����������Һ�к��������������Ϊx��
2H2O2
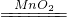 2H2O+O2��
2H2O+O2��68 32
x 0.48 g
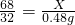 ���X=1.02g��
���X=1.02g���𣺸���Һ������˫��ˮ��ʵ������Ϊ1.02 g
�ʴ�Ϊ����1���Թܡ�����©��
��2��2KMnO4
 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2����3���Թܿ�δ��������б�������������Թ���̫����A��C��E
��4����126
��6��1��14
�������谷�е�Ԫ�ص���������66.7%
��1.02g
��������1��Ҫ��Ϥ�������������ƺ����ã���2�����ݷ���ʽ��д�����ǣ���3�����ݸ��������ȡ������ע������ͷ���װ�á��ռ�װ�õ�ѡȡ�������ǣ���4��������Է��������ļ��㡢Ԫ�������ļ��㡢Ԫ�����������ļ��㷽���ٽ��ͼ������Ϣ�ش�
������ͨ���ش���֪���˷���װ�á��ռ�װ�õ�ѡȡ���������������ȡ�����ķ�Ӧԭ������һ����������Է��������ļ��㡢Ԫ�������ļ��㡢Ԫ�����������ļ��㷽����

��ϰ��ϵ�д�
�����Ŀ