��Ŀ����
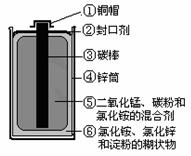
Ŀǰ���Ͼɵ�ضԻ�������Ⱦ��Խ��Խ�������ǵ����ӡ�Ϊ��ʵ�ֿɳ�����չ��ս��Ŀ�꣬������ʶ֮ʿ�����о��Ͼɵ�ص���Ч���������ü�������ͼΪ��ͨ��أ�п�̵�أ�ʾ��ͼ���������ͼʾ������
��1�������յķϾɵ�ؽ��з��룬���Եõ��ĵ����У��û�ѧʽ��ʾ����ͬ�� ���������� ������ ���л����У�д���ƣ� ��
��2��С��ͬѧ��õ����д�������Ķ������̺��Ȼ����Һ���������Ȼ���е�笠����ӡ����������ʵ�鲽�裬���������ɣ�
| ʵ�鲽�� | ʵ������ | ���ۻ���� |
| �ٰ����ɵ�أ�ȡ��̼����Χ�ĺ�ɫ��ĩ�� | ||
| ���ܽ⡢ ��ϴ�ӣ���������ƣ��� | �õ���ɫ��ĩ����ɫ����Һ�� | ��ɫ��ĩ�ijɷ�Ϊ �� |
| �۶Ժ�ɫ��ĩ���� �� | �õ�������MnO2���� | |
| �ܼ���ڢڲ��еõ�����ɫ����Һ�����Ƿ�笠����ӵķ����ǣ�
�� |
| ��ѧ����ʽΪ��
|
��1��Cu��C��Zn MnO2 NH4Cl��ZnCl2 ����
��2���ڹ��� �������̺�̿�� �����գ���¶�ڿ����м�ǿ�ȣ� ��ȡ�����Թ��У���������������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ǿ�Ҵ̼�����ζ����ʪ��ĺ�ɫʯ����ֽ����ɫ�� NH4Cl��NaOH==NaCl��H2O��NH3��
��ʾ��Ҫ�ӻ�����з�����������̺��Ȼ�泥�Ӧ���ù��˵ķ���������������õ��Լ��Ǽ���Һ��������������Һ��

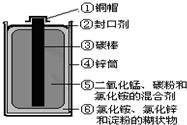
 ��2010?������Ŀǰ���Ͼɵ�ضԻ�������Ⱦ��Խ��Խ�������ǵ����ӣ�Ϊ��ʵ�ֿɳ�����չ��ս��Ŀ�꣬������ʶ֮ʿ�����о��Ͼɵ�ص���Ч���������ü�������ͼΪ��ͨ��أ�п�̵�أ�ʾ��ͼ���������ͼʾ������
��2010?������Ŀǰ���Ͼɵ�ضԻ�������Ⱦ��Խ��Խ�������ǵ����ӣ�Ϊ��ʵ�ֿɳ�����չ��ս��Ŀ�꣬������ʶ֮ʿ�����о��Ͼɵ�ص���Ч���������ü�������ͼΪ��ͨ��أ�п�̵�أ�ʾ��ͼ���������ͼʾ������ 16����ѧ�����������е���ϵ��������������⣺
16����ѧ�����������е���ϵ��������������⣺ ��2013?��ݸ��ģ��Ŀǰ���Ͼɵ�ضԻ�������Ⱦ��Խ��Խ�������ǵ����ӣ�Ϊ��ʵ�ֿɳ�����չ��ս��Ŀ�꣬������ʶ֮ʿ�����о��Ͼɵ�ص���Ч���������ü� ������ͼΪ��ͨ��أ�п�̵�أ�ʾ��ͼ���������ͼʾ�����������յķϾɵ�ؽ��з��룬���Եõ�����������
��2013?��ݸ��ģ��Ŀǰ���Ͼɵ�ضԻ�������Ⱦ��Խ��Խ�������ǵ����ӣ�Ϊ��ʵ�ֿɳ�����չ��ս��Ŀ�꣬������ʶ֮ʿ�����о��Ͼɵ�ص���Ч���������ü� ������ͼΪ��ͨ��أ�п�̵�أ�ʾ��ͼ���������ͼʾ�����������յķϾɵ�ؽ��з��룬���Եõ����������� Ŀǰ���Ͼɵ�ضԻ�������Ⱦ��Խ��Խ�������ǵ����ӣ�Ϊ��ʵ�ֿɳ�����չ��ս��Ŀ�꣬������ʶ֮ʿ�����о��Ͼɵ�ص���Ч���������ü�������ͼΪ��ͨ��أ�п�̵�أ�ʾ��ͼ���������ͼʾ�����������յķϾɵ�ؽ��з��룬���Եõ��ĵ�����
Ŀǰ���Ͼɵ�ضԻ�������Ⱦ��Խ��Խ�������ǵ����ӣ�Ϊ��ʵ�ֿɳ�����չ��ս��Ŀ�꣬������ʶ֮ʿ�����о��Ͼɵ�ص���Ч���������ü�������ͼΪ��ͨ��أ�п�̵�أ�ʾ��ͼ���������ͼʾ�����������յķϾɵ�ؽ��з��룬���Եõ��ĵ�����