��Ŀ����
С�պͰְ�����һ��ȥҰ�ͣ�����Ϊ��������һ�ּ�ʳ���ȵķ���ʳƷ����ͼ1������װ��С�շ���������һ��������װ�Ĺ������ʣ���װ��д�ţ�������ʳƷ���ȼ�������С�ն���Ϊʲô���ڶ�ʱ���ڰ�ʳ����ȵ��ϸ��¶Ȳ�����̽������Ȥ���ص�ѧУ������������ʵ���Ҷ���������չ����̽����

��Ʋ�ʵʩʵ�顢��¼ʵ����������ʵ������18�桢������ѹ�Ļ�������ɵģ��Ҳ������Ȼ��ƶ�ˮ�е��Ӱ��)��
ʵ��1����. ��һ��������þ�������ۺ��Ȼ��Ƽ���ʢ��100mLˮ�ĸ��������У��������裬ÿ50s��¼һ���¶ȣ��õ�ͼ2������a��
��. ����ͬ������þ�����ɸ�ϸ��þ˿�������þ���ظ�����ʵ�飬�õ�ͼ2������b��
��. ����ͬ������þ�۴������þ���ظ�����ʵ�飬�õ�ͼ2������c��
��1��������ʵ���֪��������ʳƷ���ȼ������ܷⱣ���ԭ����������е�________�йء������������ƣ��������仯�Ƕȿ����ñ仯����_________�ı仯��������ȡ����ȡ���
��2���۲�ͼ2�����п��Է���Ӱ��þ��ˮ��Ӧ���ʵ�������_________________��
ʵ��1����. ��һ��������þ�������ۺ��Ȼ��Ƽ���ʢ��100mLˮ�ĸ��������У��������裬ÿ50s��¼һ���¶ȣ��õ�ͼ2������a��
��. ����ͬ������þ�����ɸ�ϸ��þ˿�������þ���ظ�����ʵ�飬�õ�ͼ2������b��
��. ����ͬ������þ�۴������þ���ظ�����ʵ�飬�õ�ͼ2������c��
��1��������ʵ���֪��������ʳƷ���ȼ������ܷⱣ���ԭ����������е�________�йء������������ƣ��������仯�Ƕȿ����ñ仯����_________�ı仯��������ȡ����ȡ���
��2���۲�ͼ2�����п��Է���Ӱ��þ��ˮ��Ӧ���ʵ�������_________________��


ʵ��2����2.40gþ�ۺ�����Ϊ28.00g�����ۻ�ϣ�����ʢ��100mLˮ�ĸ��������У����Ͻ��衣�Ȼ���������ͬʱ���¶����������ͼ3��ʾ��
��3��ʵ��2�У���NaCl��������7.30gʱ��ʵ�鲻�ټ�������ԭ����_______������д�𰸱�ţ�
A����������NaCl�������ӷ�Ӧ����
B����������NaCl�ή�ͷ�Ӧ����
C���Ѵﵽˮ�ķе㣬�¶Ȳ������б仯
D��������������������ˮ���¶�
��4�������ʵ��2�м���NaCl������Ϊ3.65gʱ���������¶���߿��ܴﵽ______������д�𰸱�ţ�
A��34�� B��42�� C��50�� D��60��
��5��С�������������������ʳƷ���ȼ��������䷽������ݱ���ʵ���о��Ľ��ۣ��ж��������������_________��
A. 2.40gþ�ۡ�7.30g NaCl��28.00g����
B. 2.40gþ����7.30g NaCl��28.00g����
C. 2.40gþ�ۡ�8.76g NaCl��28.00g����
D. 2.40gþ����8.76g NaCl��28.00g����
������_________________________��
��3��ʵ��2�У���NaCl��������7.30gʱ��ʵ�鲻�ټ�������ԭ����_______������д�𰸱�ţ�
A����������NaCl�������ӷ�Ӧ����
B����������NaCl�ή�ͷ�Ӧ����
C���Ѵﵽˮ�ķе㣬�¶Ȳ������б仯
D��������������������ˮ���¶�
��4�������ʵ��2�м���NaCl������Ϊ3.65gʱ���������¶���߿��ܴﵽ______������д�𰸱�ţ�
A��34�� B��42�� C��50�� D��60��
��5��С�������������������ʳƷ���ȼ��������䷽������ݱ���ʵ���о��Ľ��ۣ��ж��������������_________��
A. 2.40gþ�ۡ�7.30g NaCl��28.00g����
B. 2.40gþ����7.30g NaCl��28.00g����
C. 2.40gþ�ۡ�8.76g NaCl��28.00g����
D. 2.40gþ����8.76g NaCl��28.00g����
������_________________________��
��1��ˮ������
��2����Ӧ��ĽӴ����
��3��C
��4��D
��5��A����Ӧ���ܳ�ֽӴ��������ϵ�������
��2����Ӧ��ĽӴ����
��3��C
��4��D
��5��A����Ӧ���ܳ�ֽӴ��������ϵ�������

��ϰ��ϵ�д�
 �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ


 ʵ�飬�õ�ͼ2������c��
ʵ�飬�õ�ͼ2������c��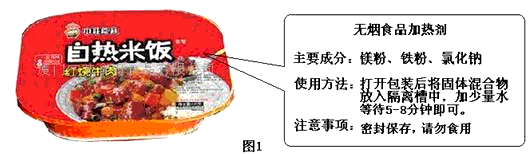

 ʵ�飬�õ�ͼ2������c��
ʵ�飬�õ�ͼ2������c��

