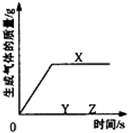
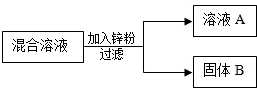
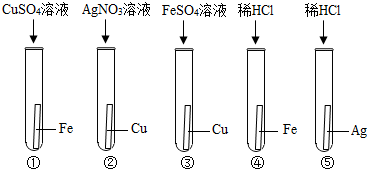
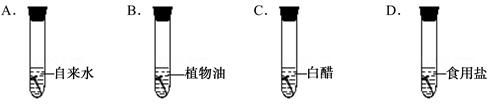
��Ŀ����
�ǽ���Ԫ�ؾ������ƽ���������Һ֮����û���Ӧ���ɣ�����Խ�ǿ�ķǽ����ɰѻ�Խ����ķǽ�����������Һ���û�������������Һ�з������з�Ӧ��C12+2NaBr=2NaCl+Br2��Br2+2KI=2KBr+I2��I2+Na2S=2NaI+S���ɴ˿��жϷǽ����Ļ�����ǿ��˳����ȷ���ǣ�������
| A��C12��I2��Br2��S | B��C12��Br2��I2��S |
| C��S��I2��Br2��Cl2 | D��C12��Br2��S��I2 |
��Ϊ��Խ�ǿ�ķǽ����ɰѻ�Խ����ķǽ�����������Һ���û���������C12+2NaBr=2NaCl+Br2��Br2+2KI=2KBr+I2��I2+Na2S=2NaI+S�������жϳ�S��C12��I2��Br2�����ǿ������˳����C12��Br2��I2��S��
��ѡB��
��ѡB��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ