��Ŀ����
 ��2012?����ģ�⣩��1������ɷ������·�Ӧ��4HNO3
��2012?����ģ�⣩��1������ɷ������·�Ӧ��4HNO3
| ||
| ���� |
O2
O2
������ı���Ӧע���ܷ⡢�ܹ⡢���������
�ܷ⡢�ܹ⡢���������
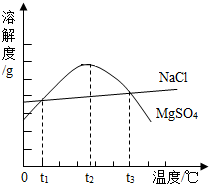
����2����ͼ��NaCl��MgSO4���ܽ�����ߣ�����˵����ȷ����
C
C
��A��ֻ����t1��ʱ��NaCl��MgSO4���ܽ�Ȳ����
B��t1�桫t3��ʱ��MgSO4���ܽ�������¶ȵ����߶�����
C��t2��ʱ��MgSO4�ı�����Һ�����ʵ������������
D����t3��ʱ��MgSO4������Һ������t2��ʱ���о���������
��������1����������Ļ�ѧ����ʽ��������غ㶨�ɵ�ʵ���������ж�X�Ļ�ѧʽ�����ݷ�Ӧ�����������ж�����ı��淽����
��2�������ܽ�����ߵ�����������������ߵĽ���Ϊ�����ʵ��ܽ����ȵ�ʱ����ͬ�¶��£��ܽ�ȵ������䱥����Һ����������Ҳ���Ծݴ��������жϣ�
��2�������ܽ�����ߵ�����������������ߵĽ���Ϊ�����ʵ��ܽ����ȵ�ʱ����ͬ�¶��£��ܽ�ȵ������䱥����Һ����������Ҳ���Ծݴ��������жϣ�
����⣺��1�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû�����������ݷ�Ӧ����ʽ4HNO3
4NO2��+X+2H2O����֪����Ӧ��ȷ�Ӧǰ����������ԭ�ӣ����Կ����ж�X�Ļ�ѧʽΪ��O2��ͬʱ���ݻ�ѧ��Ӧ��������֪�������ڹ��յ��������ֽ⣬����Ӧ���ܷ⡢�ܹⲢ�Ҵ������������
��2��A�����ܽ�������п��Կ����Ȼ��ƺ�����þ���ܽ���������������㣬�ֱ�Ϊt1���t3������t1���t3��ʱNaCl��MgSO4���ܽ�ȶ���ȣ���A˵������
B��t1�桫t2��ʱ��MgSO4���ܽ�������¶ȵ����߶�����t2�桫t3��ʱ��MgSO4���ܽ�������¶ȵ����߶���С����B˵������
C�����ܽ�������п��Կ�����t2��ʱ����þ���ܽ����������ڸ��¶��µ�MgSO4�ı�����Һ�����ʵ������������C˵����ȷ��
D��t2�桫t3��ʱ��MgSO4���ܽ�������¶ȵ����߶���С�����Խ��¶ȴ�t3�潵��t2�棬�����������ʣ���D˵������
��ѡC��
�ʴ�Ϊ��
��1��O2���ܷ⡢�ܹ⡢�����������
��2��C��
| ||
| ���� |
��2��A�����ܽ�������п��Կ����Ȼ��ƺ�����þ���ܽ���������������㣬�ֱ�Ϊt1���t3������t1���t3��ʱNaCl��MgSO4���ܽ�ȶ���ȣ���A˵������
B��t1�桫t2��ʱ��MgSO4���ܽ�������¶ȵ����߶�����t2�桫t3��ʱ��MgSO4���ܽ�������¶ȵ����߶���С����B˵������
C�����ܽ�������п��Կ�����t2��ʱ����þ���ܽ����������ڸ��¶��µ�MgSO4�ı�����Һ�����ʵ������������C˵����ȷ��
D��t2�桫t3��ʱ��MgSO4���ܽ�������¶ȵ����߶���С�����Խ��¶ȴ�t3�潵��t2�棬�����������ʣ���D˵������
��ѡC��
�ʴ�Ϊ��
��1��O2���ܷ⡢�ܹ⡢�����������
��2��C��
���������ݷ�Ӧ�Ļ�ѧ����ʽ�����÷�Ӧǰ��ԭ�����ࡢ��Ŀ���䣬���ƶϷ�Ӧ��ij���ʵķ��ӹ��ɡ�ȷ�������ʵĻ�ѧʽ�����չ����ܽ�����ߵ����弰�����ܽ�����߽����ص����⣬����ѧ��Ӧ��֪ʶ��������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2012?����ģ�⣩��ͼ��ͬѧ�Ǿ����ȵ�һ����ˮ�����ϱ���С��ͬѧ��̽����ˮ���Ƿ�����CO2������������ʵ�飺ȡ������ˮ���Թ��У��μ���ɫʯ����Һ��ʯ����Һ���ɫ��
��2012?����ģ�⣩��ͼ��ͬѧ�Ǿ����ȵ�һ����ˮ�����ϱ���С��ͬѧ��̽����ˮ���Ƿ�����CO2������������ʵ�飺ȡ������ˮ���Թ��У��μ���ɫʯ����Һ��ʯ����Һ���ɫ��