��Ŀ����
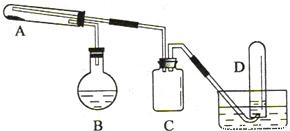
��ѧ��ȤС���ͬѧ����ʦ��ָ���£�������̼�Ͻ�������У���е������ĺ��������˲ⶨ�����ǽ���öУ����Ʒ���з��鴦��������ͬѧ��ȡ6.0g��ĩ��Ʒ�����ձ��У�Ȼ�����һ��������ϡ���ᣬ��ַ�Ӧ���ձ���ʣ�������г�������ص�ʵ�����ݼ�¼���£�

(1)�����ϱ����ݿ�֪�����Ϊ_____���ͬѧ��ʣ��ͬ�����������ݲ�������
(2)��У����Ʒ�е���������������Ϊ���٣�
(3)��ijЩ��ͬѧ�����Լ�ǡ����ȫ��Ӧ����Ӧ����Һ�����ʵ����������Ƕ��٣�
(4)���У�������⣬����ΪҪȷ�ز����Ʒ����Ԫ�ص�����������ʵ���в�����ʣ�����������Ӧ�øijɲ��������е�________������ĸ����
A������ϡ��������
B����Ӧ��ȫ���ձ����������ʵ�����
C����Ӧ��ȫ���ձ����������ʵ�������ʣ���������
(2)��У����Ʒ�е���������������Ϊ���٣�
(3)��ijЩ��ͬѧ�����Լ�ǡ����ȫ��Ӧ����Ӧ����Һ�����ʵ����������Ƕ��٣�
(4)���У�������⣬����ΪҪȷ�ز����Ʒ����Ԫ�ص�����������ʵ���в�����ʣ�����������Ӧ�øijɲ��������е�________������ĸ����
A������ϡ��������
B����Ӧ��ȫ���ձ����������ʵ�����
C����Ӧ��ȫ���ձ����������ʵ�������ʣ���������
(1)3
(2)���ݱ������ݿ�֪��Ʒ����������Ϊ6.0g-0.4g=5.6g
����������������=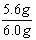 �� 100%=93.3%
�� 100%=93.3%
(3)�⣺������H2������Ϊx��
Fe+H2SO4==FeSO4+H2��
56����������152��2
5.6g��������y����x
 ��x=0.2g
��x=0.2g
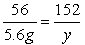 ��y= 15.2g
��y= 15.2g
��Ӧ����Һ�����ʵ���������Ϊ ��100%=33.5%
��100%=33.5%
(4)C
(2)���ݱ������ݿ�֪��Ʒ����������Ϊ6.0g-0.4g=5.6g
����������������=
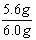 �� 100%=93.3%
�� 100%=93.3%(3)�⣺������H2������Ϊx��
Fe+H2SO4==FeSO4+H2��
56����������152��2
5.6g��������y����x
 ��x=0.2g
��x=0.2g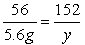 ��y= 15.2g
��y= 15.2g��Ӧ����Һ�����ʵ���������Ϊ
 ��100%=33.5%
��100%=33.5%(4)C

��ϰ��ϵ�д�
�����Ŀ
��ѧ��ȤС���ͬѧ����ʦ��ָ���£�������̼�Ͻ�������У���е������ĺ��������˲ⶨ�����ǽ���öУ����Ʒ���з��鴦��������ͬѧ��ȡ6.0g��ĩ��Ʒ�����ձ��У�Ȼ�����һ��������ϡ���ᣬ��ַ�Ӧ���ձ���ʣ�������г�������ص�ʵ�����ݼ�¼�����
��1�������ϱ����ݿ�֪�����Ϊ ���ͬѧ��ʣ��������������ݲ�������
��2����У����Ʒ�е���������������Ϊ���٣�
��3����ijЩ��ͬѧ�����Լ�ǡ����ȫ��Ӧ����Ӧ����Һ�����ʵ����������Ƕ��٣�
��4�����У�������⣬����ΪҪȷ�ز����Ʒ����Ԫ�ص�����������ʵ���в�����ʣ�����������Ӧ�øijɲ��������е� ������ĸ����
A������ϡ��������
B����Ӧ��ȫ���ձ����������ʵ�����
C����Ӧ��ȫ���ձ����������ʵ�������ʣ�����������
| ����� | ��Ʒ����/g | ����ϡ��������/g | ʣ���������/g |
| 1 | 6.0 | 40.0 | 0.4 |
| 2 | 6.0 | 40.0 | 0.4 |
| 3 | 6.0 | 50.0 | 0.6 |
| 4 | 6.0 | 50.0 | 0.4 |
��2����У����Ʒ�е���������������Ϊ���٣�
��3����ijЩ��ͬѧ�����Լ�ǡ����ȫ��Ӧ����Ӧ����Һ�����ʵ����������Ƕ��٣�
��4�����У�������⣬����ΪҪȷ�ز����Ʒ����Ԫ�ص�����������ʵ���в�����ʣ�����������Ӧ�øijɲ��������е�
A������ϡ��������
B����Ӧ��ȫ���ձ����������ʵ�����
C����Ӧ��ȫ���ձ����������ʵ�������ʣ�����������