��Ŀ����
̼�ڵؿ��еĺ������ߣ������Ļ����������ڶ࣬���ҷֲ����㡣
������ͼ����ѧ֪ʶ�ش��������⣺
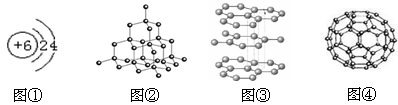
��1����ͼ�����ڽ��ʯ�ṹ���� ����дͼ��ţ���ͼ��Ҳ��̼��һ�ֵ��ʣ��仯ѧʽΪ ��ͼ��Ϊ̼Ԫ�ص�ԭ�ӽṹʾ��ͼ��������̼�Ļ�ѧ����________������á������á�����
��2����֪X���ж��Ҳ�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮������ͼת����ϵ��
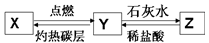
��X�Ļ�ѧʽΪ___________��
��Y��ʯ��ˮ��Ӧ����Z�Ļ�ѧ����ʽΪ_________��
������X��Y������Ԫ����ͬ�������ǵĻ�ѧ���ʲ�ͬ����ԭ����___________.
��3����̼��������ͭ��Ϻ��ǿ�ȣ�������Ӧ�Ļ�ѧ����ʽΪ____________��
��4���ڿ�ѧ�����CO2�ǿ������õ���Ҫ��Դ����һ�������£�CO2�ͽ����Ʒ�Ӧ������ȡ���ʯ����Ӧ�ķ���ʽ��CO2+4Na C�����ʯ��+2Na2O �������92g�����������Ͽ����Ƶú�̼96%�Ľ��ʯ��������
C�����ʯ��+2Na2O �������92g�����������Ͽ����Ƶú�̼96%�Ľ��ʯ��������

������ͼ����ѧ֪ʶ�ش��������⣺
��1����ͼ�����ڽ��ʯ�ṹ���� ����дͼ��ţ���ͼ��Ҳ��̼��һ�ֵ��ʣ��仯ѧʽΪ ��ͼ��Ϊ̼Ԫ�ص�ԭ�ӽṹʾ��ͼ��������̼�Ļ�ѧ����________������á������á�����
��2����֪X���ж��Ҳ�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮������ͼת����ϵ��

��X�Ļ�ѧʽΪ___________��
��Y��ʯ��ˮ��Ӧ����Z�Ļ�ѧ����ʽΪ_________��
������X��Y������Ԫ����ͬ�������ǵĻ�ѧ���ʲ�ͬ����ԭ����___________.
��3����̼��������ͭ��Ϻ��ǿ�ȣ�������Ӧ�Ļ�ѧ����ʽΪ____________��
��4���ڿ�ѧ�����CO2�ǿ������õ���Ҫ��Դ����һ�������£�CO2�ͽ����Ʒ�Ӧ������ȡ���ʯ����Ӧ�ķ���ʽ��CO2+4Na
 C�����ʯ��+2Na2O �������92g�����������Ͽ����Ƶú�̼96%�Ľ��ʯ��������
C�����ʯ��+2Na2O �������92g�����������Ͽ����Ƶú�̼96%�Ľ��ʯ����������1���� C60 ������
��2���� CO ��CO2+Ca��OH��2=CaCO3��+H2O �۷��ӵĹ��ɲ�ͬ
��3�� C+2CuO 2Cu+ CO2��
2Cu+ CO2��
��4�� 12.5g
��2���� CO ��CO2+Ca��OH��2=CaCO3��+H2O �۷��ӵĹ��ɲ�ͬ
��3�� C+2CuO
 2Cu+ CO2��
2Cu+ CO2����4�� 12.5g
�����������1�����ʯ��Ӳ�ȴ��Ǣ� ��ͼ�ܽṹ��״������Ӧ��C60
��2����X���ж��Ҳ�����ˮ�����壬��ôX��һ����̼��һ����̼ȼ�����ɵ��Ƕ�����̼����Y�Ƕ�����̼��������̼��ʯ��ˮ��Ӧ���ɵ���̼��ơ�
��3��̿�۾��л�ԭ�ԣ��ܻ�ԭ����ͭ����ͭ�Ͷ�����̼��
��4�� ���û�ѧ����ʽ��������ʯ�������ȣ���Ͻ����Ƶ�����������������Ľ��ʯ�����������ý��ʯ���������Դ��ȣ����Ǻ����ʵĵĽ��ʯ��������
�裺̼������ΪX
CO2+4Na
 C�����ʯ��+2Na2O
C�����ʯ��+2Na2O4��23 12
92g X
92/12=92g/X
X=12g
12g/96%=12.5g
�𣺿����Ƶú�̼96%�Ľ��ʯ12.5g

��ϰ��ϵ�д�
�����Ŀ
