��Ŀ����
���������ಢ�������ã��Ƚ�Լ��Ȼ��Դ���ּ��ỷ����Ⱦ��
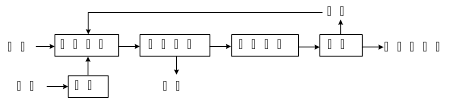
��1���ϲ������ϸֽ�Ͼ���̥�ȷ������У��ӷ���ĽǶ������л����������
��2�����յķ��������У���һ�����ͨ���������ڵķ�����ʹ�����������Ϊ������Ʒ���ù��̷����ı仯����
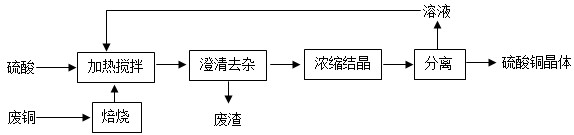
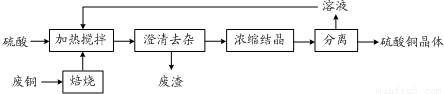
��3���ϼ��õ��������Եȶຬ�н𡢲��ȹ��ؽ�����һ��������ݻ���յȷ���ֱ�ӻ��գ����������˽𡢲���
��4�����ڲ��ɻ��յ���������ʳ��������������������е��л�����зֽ⣬��õĺ�����Ԫ�صĻ������ũҵ�����Ͽ�����
��5�����ڽ�����Դ�Ķ�ȱ���������һ���������Ľ��飺
��1���ϲ������ϸֽ�Ͼ���̥�ȷ������У��ӷ���ĽǶ������л����������
�Ͼ���̥
�Ͼ���̥
����2�����յķ��������У���һ�����ͨ���������ڵķ�����ʹ�����������Ϊ������Ʒ���ù��̷����ı仯����
����
����
�仯����3���ϼ��õ��������Եȶຬ�н𡢲��ȹ��ؽ�����һ��������ݻ���յȷ���ֱ�ӻ��գ����������˽𡢲���
Au��Ptͨ�������ᡢ������Ӧ
Au��Ptͨ�������ᡢ������Ӧ
���ʣ���4�����ڲ��ɻ��յ���������ʳ��������������������е��л�����зֽ⣬��õĺ�����Ԫ�صĻ������ũҵ�����Ͽ�����
����
����
�������ɻ����÷��չ��մ��������磬�ù����л�ѧ��ת��Ϊ��
��
�ܣ�����ת��Ϊ���ܣ���5�����ڽ�����Դ�Ķ�ȱ���������һ���������Ľ��飺
�Ͼɽ�������������
�Ͼɽ�������������
����������1�������������ǽ������ϣ��ֽ����ڽ������ϣ��Ͼ���̥�����л������
��2��ͨ���������ڵķ�����ʹ�����������Ϊ������Ʒ���˹���û�����������ɣ�������������仯����3���𡢲��ȹ��ؽ�������ѧ�����ȶ���һ��������ݻ���յȷ���ֱ�ӻ��գ����������˽𡢲���Au��Ptͨ�������ᡢ������Ӧ�����ʣ�
��4����õĺ�����Ԫ�صĻ�������ڻ����еĵ��ʣ����÷��չ��մ��������磬�ù����л�ѧ��ת��Ϊ���ܣ�����ת��Ϊ���ܣ�
��5���ӽ����ķ��������������ü����ɺ�Ѱ���µĴ���Ʒ��Щ����ȥ���ǣ�
��2��ͨ���������ڵķ�����ʹ�����������Ϊ������Ʒ���˹���û�����������ɣ�������������仯����3���𡢲��ȹ��ؽ�������ѧ�����ȶ���һ��������ݻ���յȷ���ֱ�ӻ��գ����������˽𡢲���Au��Ptͨ�������ᡢ������Ӧ�����ʣ�
��4����õĺ�����Ԫ�صĻ�������ڻ����еĵ��ʣ����÷��չ��մ��������磬�ù����л�ѧ��ת��Ϊ���ܣ�����ת��Ϊ���ܣ�
��5���ӽ����ķ��������������ü����ɺ�Ѱ���µĴ���Ʒ��Щ����ȥ���ǣ�
����⣺��1�������������ǽ������ϣ��ֽ����ڽ������ϣ��Ͼ���̥�����л������
�ʴ�Ϊ���Ͼ���̥��
��2��ͨ���������ڵķ�����ʹ�����������Ϊ������Ʒ���˹���û�����������ɣ�������������仯��
�ʴ�Ϊ��������
��3���𡢲��ȹ��ؽ�������ѧ�����ȶ���һ��������ݻ���յȷ���ֱ�ӻ��գ����������˽𡢲���Au��Ptͨ�������ᡢ������Ӧ�����ʣ�
�ʴ�Ϊ��Au��Ptͨ�������ᡢ������Ӧ��
��4����õĺ�����Ԫ�صĻ�������ڻ����еĵ��ʣ����÷��չ��մ��������磬�ù����л�ѧ��ת��Ϊ���ܣ�����ת��Ϊ���ܣ�
�ʴ�Ϊ�����ϣ��ȣ�
��5�����ڽ�����Դ�ı��������ǿ��Դ����¼������������
ÿ����кܶ��������ʴ���˷ѵ�������Ҫ��ֹ������ʴ����ֻ��������ã�������Դ�����п������������ڿ������������µĹ����������˷ѵ�������������Ѱ�ҵͼ۽����Ͻ�ϲ��ϴ���۽����Ľ��������
�ʴ�Ϊ���Ͼɽ������������ã��𰸺������ɣ���
�ʴ�Ϊ���Ͼ���̥��
��2��ͨ���������ڵķ�����ʹ�����������Ϊ������Ʒ���˹���û�����������ɣ�������������仯��
�ʴ�Ϊ��������
��3���𡢲��ȹ��ؽ�������ѧ�����ȶ���һ��������ݻ���յȷ���ֱ�ӻ��գ����������˽𡢲���Au��Ptͨ�������ᡢ������Ӧ�����ʣ�
�ʴ�Ϊ��Au��Ptͨ�������ᡢ������Ӧ��
��4����õĺ�����Ԫ�صĻ�������ڻ����еĵ��ʣ����÷��չ��մ��������磬�ù����л�ѧ��ת��Ϊ���ܣ�����ת��Ϊ���ܣ�
�ʴ�Ϊ�����ϣ��ȣ�
��5�����ڽ�����Դ�ı��������ǿ��Դ����¼������������
ÿ����кܶ��������ʴ���˷ѵ�������Ҫ��ֹ������ʴ����ֻ��������ã�������Դ�����п������������ڿ������������µĹ����������˷ѵ�������������Ѱ�ҵͼ۽����Ͻ�ϲ��ϴ���۽����Ľ��������
�ʴ�Ϊ���Ͼɽ������������ã��𰸺������ɣ���
�����������㿼���˻�ѧ����ϡ������仯�ͻ�ѧ�仯�ı�𡢽��������ʡ��Ͻ�����ʵȣ������֪ʶ��Ƚ϶࣬Ҫ��ǿ���䣬����Ӧ�ã���������Ҫ������ѡ�����������У�

��ϰ��ϵ�д�
 С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д� ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
�����Ŀ