��Ŀ����
����Ŀ����10�֣�ij����С���ͬѧ����ͼ��ʾװ�ã�����������ĩ(����������)��Ʒ����ʵ�顣ͼ������̨��װ������ȥ����ش��й����⣺
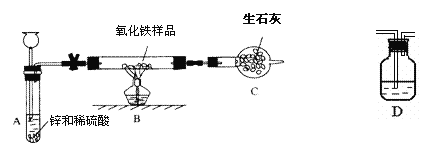
��1��ʵ��Ŀ�ģ��ⶨ ��
��2������С���ͬѧ��ʵ�����ʱ��Ӧע�����ǰ��ͨһ���������Ŀ���� ����Ӧ���Ҫ����ͨ�������Թ���ȴ����Ϊ�� ��
��3��ʵ��������йػ�ѧ����ʽ���±���H2�Ļ�ѧ������CO���ƣ�������ɱ���
ʵ������ | ��ѧ����ʽ |
A�з�Ӧ�����������ݣ� | A����Zn+ H2SO4= ZnSO4+H2���� |
B�� ��ĩ���ɫ�� | B�У� �� |
��4��ʵ�����ݼ�¼�ʹ����������淶�IJ�������ȷ�ij������õ�������������(B�з�Ӧ��ȫ��C��������ȫ)��
��Ӧǰ | ��������ȫ��Ӧ�� | |
���� | B�������ܵ�����32.4�� ��������Ʒ������10.0�� | B�������ܺ��ڹ������ʵ�����40.0�� |
���� | C������ܺ���ʯ������40��O�� | C������ܺ��ڹ������ʵ�����43.0�� |
ͨ�����㣬ͬѧ�Ƿ��֣��õõ������������������Ʒ��������������������ͬ�������Ӧ��ѡ�� �����ݡ���Ʒ��������������������_______��
��5����������ۣ�
�������ۣ�ͬѧ�Ƕ�װ�ý����˸Ľ����� ����2��װ�ô��ţ�֮��������һ����ͼ��ʾ��Dװ�ã��ٴ�ʵ����õõ������������������Ʒ������������������������ͬ��ͼDװ�����Һ���� ��
���𰸡���1���ⶨ��������ĩ��Ʒ������������������
��2���ž���������ֹ����ʱ������ը�� Ϊ�˷�ֹ��������ʹ�����±�����
��3������ɫ 3H2 + Fe2O3 ���� 2Fe + 3 H2O
��4���� 80%
��5��A��B ŨH2SO4
��������
�����������1��ʵ��Ŀ�ģ��ⶨ�ⶨ��������ĩ��Ʒ������������������
��2�������������п�ȼ�ԣ�����������ʱ�������������¿��ܷ�����ը������ʵ�����ʱ��Ӧע�����ǰ��ͨһ���������Ŀ�����ž���������ֹ����ʱ������ը����Ӧ���Ҫ����ͨ�������Թ���ȴ����Ϊ����Ϊ�˷�ֹ��������ʹ�����±�����
��3��װ��A�����ɵ��������л�ԭ�ԣ�������������Ӧ����B�е�ʵ������ʱ������ɫ��ĩ���ɫ��������Ӧ�Ļ�ѧ����ʽ��3H2 + Fe2O3 ���� 2Fe + 3 H2O
��4���õõ������������������Ʒ��������������������ͬ��ԭ����װ��C�е���ʯ�Ҳ���������װ��B�����ɵ�ˮ����������ԭ������ˮ�֣�������ʹˮ������ƫ�Ӷ��������������������������Ҳƫ��Ӧ��ѡ���������������㣬���ݻ�ѧ��Ӧ��3H2 + Fe2O3 ���� 2Fe + 3 H2O����Ӧǰ��������ΪFe2O3�е���Ԫ�ص���������=32.4g+10g-40g=2.4g,�ٸ���Ԫ������=���ʵ�������Ԫ�ص������������ʿ���������������Ϊx����ʽΪ��2.4g=x��48/160��100%��x=8g������Ʒ������������������=8g/10g��100%=80%
��5����Ӧ��װ�ý����˸Ľ�����A��B֮��������һ����ͼ��ʾ��Dװ�ã����������и�����ٴ�ʵ����õõ������������������Ʒ������������������������ͬ��ͼDװ�����Һ���� ŨH2SO4