��Ŀ����
����Ŀ����ɽ��Ա���õ���Դ���⻯��(CaH2)���壬������ˮ��Ӧ������������[��ѧʽΪCa(OH)2]��������������ȼ��֮�衣��ش���������:
��1���⻯������Ԫ�صĻ��ϼ�Ϊ________��
��2��д���⻯����ˮ��Ӧ�Ļ�ѧ����ʽ:_______��
��3��������г��ᣬ�������������ũ���Ŀ��֮һ����ũ��������������ˮ��ClO2����һ������ˮ�����������������Ҳ���ClO2����Cl2��������ˮ����������ش��������⣺
��ClO2������Ϊ ______��
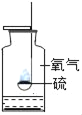
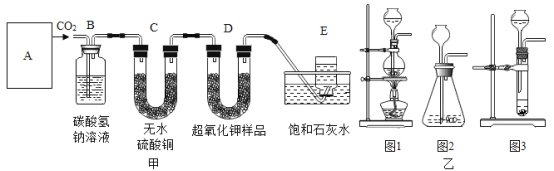
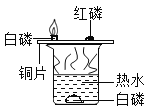
����ȡClO2���۹�����ͼ��ʾ��

���ݷ�Ӧ���۹���ͼд����Ӧ�Ļ�ѧ����ʽ______��
���𰸡�-1 CaH2+2H2O=Ca(OH)2+2H2�� �������� Cl2+2NaClO2=2NaCl+2ClO2
��������
��1�����⻯��(CaH2)����Ԫ�صĻ��ϼ�Ϊx�����ݻ��������������ϼ۴�����Ϊ0�У���+2��+x��2=0��x=-1�����⻯������Ԫ�صĻ��ϼ�Ϊ-1�ۣ�
��2���������Ϣ��֪���⻯�ƺ�ˮ��Ӧ�����������ƺ���������ѧ����ʽΪ��CaH2+2H2O= Ca(OH)2+2H2����
��3���ٸ��ݻ�ѧʽ�Ķ�����ClO2������Ϊ�������ȣ�
����ͼ��֪����Ӧ��ΪCl2��NaClO2��������ΪClO2 ��NaCl������ͼ������Ŀ������ƽ���÷�Ӧ�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2NaCl+2ClO2��

����Ŀ��(1)��ͼ�ĶԱ�ʵ��̽�����������������֤�������μ��˷�Ӧ����Ҫ����ʵ����____________(�����)

(2)ů����������������������ų�������ԭ����ijС��ͬѧ��Ʋ�����ʵ�飬̽��Ӱ�����������ʵ�����
[��������]ů���ķ��Ȳ�����Ҫ�����ۡ�����̿��ʳ�Ρ�ˮ�����ʡ�
[�������]�ٻ���̿�ܼӿ�������������ʡ���ʳ���ܼӿ�������������ʡ�
[����ʵ��]�±���2g���ۡ�5gˮ�벻ͬ������̿��ʳ�ξ��Ȼ�Ϻ���10�������¶�������ʵ���¼��
ʵ���� | ����̿/g | ʳ��/g | �¶�����ֵ/��C |
1 | 0 | 0 | 0.1 |
2 | 0.2 | 0 | 22.0 |
3 | X | 0.1 | 3.1 |
4 | 0.2 | 0.1 | 56.3 |
[���������]�ٱ���X��ֵΪ_________________��
�ڶԱ�ʵ��1��3���ó��Ľ�����_________________��
�����ó�����������̿��ʳ��ͬʱ���ڻ����ȼӿ�����������������Ҫ�Ա�_____________(��ʵ����)��
[��˼������]����˵����ȷ����_________________��
A.ů����ʹ��ǰҪ�ܷⱣ��
B.ů��ʹ�ú�ʣ������Ի�������
C.�����������ԭ�������ۻ�������ʳƷ����
����Ŀ��������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ���õ��ǣ� ��
ʵ �� װ �� |
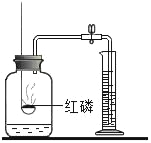
����������ȼ�� |
�ⶨ�������������� |
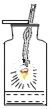
�ⶨ�������������� |
��˿��������ȼ�� |
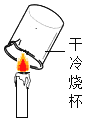
���� | ����ƿ�е�ˮ�� ���շų������� | ����ƿ�е�ˮ�� ��������հ��� | ��Ͳ�е�ˮ�� ͨ��ˮ����ı仯�ó�������� | ����ƿ�е�ˮ�� ��ȴ�����������ֹ����ƿը�� |
A | B | C | D |
A. AB. BC. CD. D