��Ŀ����
��ͼ��ʵ���ҳ��õ�ʵ��װ�ã���ش��й����⣺

��1��װ��C�ɵ��ܡ� �� �������������ƣ���ɡ�
��2��ʵ������H2O2��MnO2��ȡO2�Ļ�ѧ����ʽΪ ��ѡ�õķ���װ���� ������ţ���ͬ��������KClO3��MnO2��ȡO2 ʱ����ѡ�õķ���װ���� ��
��3��ʵ������ȡCO2�Ļ�ѧ����ʽΪ �����Ƶõ�CO2�л���HCl��H2O���ᴿʱ��NaOH�����ȥ���ʣ�����Ϊ���ַ��� ������С������С����������� ��
��4��ʵ������ȡH2Ӧѡ�õ��ռ�װ���� �� ��

��1��װ��C�ɵ��ܡ� �� �������������ƣ���ɡ�
��2��ʵ������H2O2��MnO2��ȡO2�Ļ�ѧ����ʽΪ ��ѡ�õķ���װ���� ������ţ���ͬ��������KClO3��MnO2��ȡO2 ʱ����ѡ�õķ���װ���� ��
��3��ʵ������ȡCO2�Ļ�ѧ����ʽΪ �����Ƶõ�CO2�л���HCl��H2O���ᴿʱ��NaOH�����ȥ���ʣ�����Ϊ���ַ��� ������С������С����������� ��
��4��ʵ������ȡH2Ӧѡ�õ��ռ�װ���� �� ��
��
 ��1������ƿ ˮ��
��1������ƿ ˮ��
(2) 2H2O2 ��2H2O + O2�� B A
��3��CaCO3+2HCl ��CaCl2+H2O+CO2�� ������ ��ΪCO2Ҳ����NaOH��Ӧ
��4) C D
 ��1������ƿ ˮ��
��1������ƿ ˮ�� (2) 2H2O2 ��2H2O + O2�� B A
��3��CaCO3+2HCl ��CaCl2+H2O+CO2�� ������ ��ΪCO2Ҳ����NaOH��Ӧ
��4) C D
��1������ƿ�ռ������װ�ã�ˮ��ʱʢˮ��װ�ã��ʴ�Ϊ������ƿ��ˮ��
��2�������˫��ˮ�������Ͳ���Ҫ���ȣ�����ø�����ػ����������������Ҫ���ȣ����������ڶ�������������������������ˮ���������ʴ�Ϊ��2H2O2
 2H2O+O2����B��A
2H2O+O2����B��A��3��ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���������̼��ʹ�����ʯ��ˮ����ǣ��ʴ�Ϊ��B��CaCO3+2HCl=CaCl2+H2O+CO2���������ʯ��ˮ�����.
��Ҫ���������������ơ��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д���ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

��ϰ��ϵ�д�
�����Ŀ

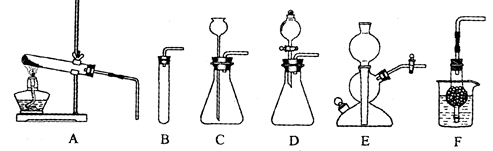

 ��װ����50mLˮ����ʵ�����������Щ���ã�_____����д��ţ�
��װ����50mLˮ����ʵ�����������Щ���ã�_____����д��ţ�
