��Ŀ����
10����֪ʶ�Ĺ�����������ѧϰ��Ȼ��ѧ����Ҫ��������1����������������ѧ��Ӧ����2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O ��2CO+O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2 ��3Fe+2O2$\frac{\underline{\;��ȼ\;}}{\;}$Fe3O4
ͨ���ȽϿɷ�����������������֮����
��һ���ӻ�ѧ��Ӧ���Ϳ������ǻ��Ϸ�Ӧ��
���������������������������
����д��������������Ҫ���һ����ѧ����ʽ��S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2 ��
���� ��1���Ա���������ʽ���ҳ����Ƶ㲢�ش�
��2��������һ�������Ҫ����д����ʽ��
��� �⣺��һ��ͨ���ȽϷ������Ƕ��ǻ��Ϸ�Ӧ��
������������������������������������μӷ�Ӧ�����������ǵ�ȼ�ȣ�
�������������Ļ��У�S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2
�ʴ�Ϊ�����Ϸ�Ӧ������������������������S+O2$\frac{\underline{\;��ȼ\;}}{\;}$SO2
���� ��ѧ����ʽ������Ҫ�Ļ�ѧ����֮һ��Ӧ�ù㷺���ǿ�����ص���ȵ㣬�ر�����Ϣ����ʽ����д���ڿ�����ռ����Ҫ��λ��

��ϰ��ϵ�д�
�����Ŀ
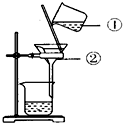 ��ͼ��ʾ��
��ͼ��ʾ��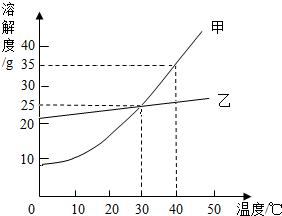 ��ͼ�Ǽס������������ʵ��ܽ�����¶ȱ仯������ͼ���ݴ����ܻش��������⣺
��ͼ�Ǽס������������ʵ��ܽ�����¶ȱ仯������ͼ���ݴ����ܻش��������⣺