��Ŀ����
���������������ݿ���ʴ��ϵ����20����ǰ�ѱ������˽⣮Ϊ���о����ǵĹ�ϵ����ѧ����һ������Ȼ������ĺ����������������1000����ͯ�����ݼ�飬����һ��Ϊ5���ͯ�������ڣ�����һ��Ϊ12���ͯ��ͼ����ʾ��ͯ���õġ����˵Ļ�������������

������ͯ��ÿ��ˮ�к�������0��25�ˣ��ϰ���ͯ��ÿ��ˮ�к�������1��40�ˣ�������ij�˶������ͼ������������������Ϊ����ȷ����
- A.5������12����������
- B.ʹ��һ�������������������������ڷ�ֹ����
- C.������ͯ���ϰ���ͯ��������
- D.�����ﺬ��Խ�߶�����Ŀ���ʴ����Խǿ
D
���������ȷ���ͼ�α�ʾ��������о��Ķ���Ȼ��Ա�ͼ�εIJ�ͬ�������ɵó����ۣ�ͬʱҪ����й����ʶ���з�����
���A���Ա�ǰ������ͼ�������Բ��ܱ��������ϰ��Ķ���5���ͯ�ĸ��õġ����˵��������࣬����ѡ����ȷ��
B������Ϊ�����к��еķ�Ԫ�ؾ��з�ȣ�̳����ã��������Ŷ�ͯ�����������ʼʹ������ˢ�������������ı���Ҳ���ͣ�������ȷ��
C���Ա�����ͼ�Σ��ɷ��ֲ����������С�����DZ����Ķ�ͯ���õġ����˵��������࣬������ȷ��
D����Ԫ��������������Ԫ�أ������ڷ�ֹȣ�ݣ����������룬�÷������ͷ��Dz�������������룬���Դ���
��ѡD
������������Ҫ����ѧ������ͼ������ȡ��Ϣ��������ͬʱҲ������ȷԪ�ض����彡�������ã����벻���������������������彡����
���������ȷ���ͼ�α�ʾ��������о��Ķ���Ȼ��Ա�ͼ�εIJ�ͬ�������ɵó����ۣ�ͬʱҪ����й����ʶ���з�����
���A���Ա�ǰ������ͼ�������Բ��ܱ��������ϰ��Ķ���5���ͯ�ĸ��õġ����˵��������࣬����ѡ����ȷ��
B������Ϊ�����к��еķ�Ԫ�ؾ��з�ȣ�̳����ã��������Ŷ�ͯ�����������ʼʹ������ˢ�������������ı���Ҳ���ͣ�������ȷ��
C���Ա�����ͼ�Σ��ɷ��ֲ����������С�����DZ����Ķ�ͯ���õġ����˵��������࣬������ȷ��
D����Ԫ��������������Ԫ�أ������ڷ�ֹȣ�ݣ����������룬�÷������ͷ��Dz�������������룬���Դ���
��ѡD
������������Ҫ����ѧ������ͼ������ȡ��Ϣ��������ͬʱҲ������ȷԪ�ض����彡�������ã����벻���������������������彡����

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ
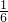 �������ޣ��ر����й��������ճ�������Ԫ�ط�����
�������ޣ��ر����й��������ճ�������Ԫ�ط�����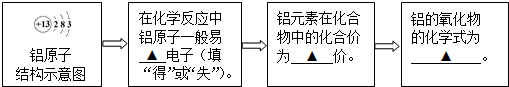
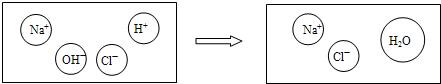

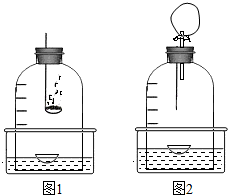 ����þ���ܡ����Ķ�ȥ��
����þ���ܡ����Ķ�ȥ��
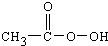 �������������
�������������