��Ŀ����
���ž��õķ�չ��������Ϊ�����������ע�����⡣��1��Ϊ�˽��ʹ����ж�����̼�ĺ�������ѧ�������뾡�취�û�ѧ��Ӧ���ֶ�ʹ������̼�����Ϊ����������������������ڸ�ѹ�������¶������£�CO2��H2���Ը�Ч�ϳɼ���(HCOOH)����ʵ���˹�ҵ���������÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��2��Ŀǰʹ��Ĥ���뷨�ӿ����з����CO2�����������CO2������________ (���������ѧ��)�仯��
��3��2011��3�£��ձ�������һ�˵�վ�����˷���й©����о�ۻ��Ǻ˵��¹��������ص���̬�������һ�˵�վ���鷴Ӧ��о�ų����������Ӵ����������������ҷ�Ӧ������������ը����д��������ըʱ�Ļ�ѧ����ʽ__________��
��4����������SO2������Ⱦʱ����ͨ���ɻ�����X��ĩ��ʹ�����е�SO2�������Խ��ͣ��ù��̷����ķ�Ӧ��2X+2SO2+O2 2CaSO4+2CO2��X�Ļ�ѧʽ��__________��
��5�����������ʶ������������Զ�����ҵ�е�һ����ģ��(��ͼ��ʾ)�����������ں�����Ϫˮ����Ⱦ���ơ�������ˮ�Ķ������辧������Ƴ�С���������ڲ��Ĵ������£�˫��ˮ�ֽ⣬ͬʱʹһ������������������һ�������������ϡ�����������Ϣ�жϣ������й�˵���������__________��

| A�����������������к��еĹ衢��ԭ�Ӹ�����Ϊ1��2 |
| B�����������ʶ����壬������С��������Χ |
| C��˫��ˮ�ֽ�ʱ�����������Ǵ��� |
| D����Ϊ�������辧������ˮ��Ӧ���ܽ⣬���Ը�������������������ˮ��Ⱦ�Ŀ��� |
��1��CO2+H2  HCOOH
HCOOH
��2������
��3��2H2+O2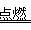 2H2O
2H2O
��4��CaCO3
��5��CD (2�֣���ѡ��©ѡû��)����:
��
 HCOOH
HCOOH ��2������
��3��2H2+O2
 2H2O
2H2O��4��CaCO3
��5��CD (2�֣���ѡ��©ѡû��)����:
��

��ϰ��ϵ�д�
�����Ŀ
 ����������������ڸ�ѹ�������¶������£�CO2��H2���Ը�Ч�ϳɼ��ᣨHCOOH������ʵ���˹�ҵ���������÷�Ӧ�Ļ�ѧ����ʽΪ
����������������ڸ�ѹ�������¶������£�CO2��H2���Ը�Ч�ϳɼ��ᣨHCOOH������ʵ���˹�ҵ���������÷�Ӧ�Ļ�ѧ����ʽΪ
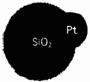 ��5�����������ʶ������������Զ�����ҵ�е�һ����ģ��(��ͼ��ʾ)�����������ں�����Ϫˮ����Ⱦ���ơ�������ˮ�Ķ������辧������Ƴ�С���������ڲ��Ĵ������£�˫��ˮ�ֽ⣬ͬʱʹһ������������������һ�������������ϡ�����������Ϣ�жϣ������й�˵����������__________��
��5�����������ʶ������������Զ�����ҵ�е�һ����ģ��(��ͼ��ʾ)�����������ں�����Ϫˮ����Ⱦ���ơ�������ˮ�Ķ������辧������Ƴ�С���������ڲ��Ĵ������£�˫��ˮ�ֽ⣬ͬʱʹһ������������������һ�������������ϡ�����������Ϣ�жϣ������й�˵����������__________��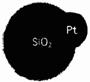 ��5�����������ʶ������������Զ�����ҵ�е�һ����ģ��(��ͼ��ʾ)�����������ں�����Ϫˮ����Ⱦ���ơ�������ˮ�Ķ������辧������Ƴ�С���������ڲ��Ĵ������£�˫��ˮ�ֽ⣬ͬʱʹһ������������������һ�������������ϡ�����������Ϣ�жϣ������й�˵����������
��5�����������ʶ������������Զ�����ҵ�е�һ����ģ��(��ͼ��ʾ)�����������ں�����Ϫˮ����Ⱦ���ơ�������ˮ�Ķ������辧������Ƴ�С���������ڲ��Ĵ������£�˫��ˮ�ֽ⣬ͬʱʹһ������������������һ�������������ϡ�����������Ϣ�жϣ������й�˵���������� 2CaSO4+2CO2��X�Ļ�ѧʽ�ǣ�
2CaSO4+2CO2��X�Ļ�ѧʽ�ǣ�