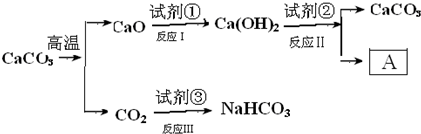
��Ŀ����
 ��2008?ï����ij��ѧ��ȤС��Ϊ�˲ⶨ��ͭ��ͭп�Ͻ𣩵���ɣ�ͬѧ�dz�ȡ10g��Ʒ��Ͷ�뵽װ��100g������������Ϊ9.8% ��ϡ������ձ��У�ǡ����ȫ��Ӧ���õ��ձ���ʣ���������Һ�Ͳ�����壩������Ϊ109.8g��
��2008?ï����ij��ѧ��ȤС��Ϊ�˲ⶨ��ͭ��ͭп�Ͻ𣩵���ɣ�ͬѧ�dz�ȡ10g��Ʒ��Ͷ�뵽װ��100g������������Ϊ9.8% ��ϡ������ձ��У�ǡ����ȫ��Ӧ���õ��ձ���ʣ���������Һ�Ͳ�����壩������Ϊ109.8g����ش��������⣺
��1�����������غⶨ�ɿɵ���������
0.2g
0.2g
g����2�����100gϡ��������������������Ϊ98%��Ũ����ϡ�Ͷ��ɵģ�����Ҫ��
90
90
gˮϡ��Ũ���ᣮ��3��ͨ�����㣬�����±ߵ������л����������������õ�ϡ����ı仯����ͼ��
��4�����㷴Ӧ��������Һ������������������д��������̣������ȷ��0.1%��
��������1�������ձ��������ļ������������ɵ�������������2���������Ũ���������������������100g��ȥŨ����ľ��Ǽ�ˮ����������3����������������������������������ֵ��ͼ����4�����������������п�����������ɵ�����п���������������Ӧ������Һ������������п���������Է�Ӧ��������Һ�������ٷ�֮�ټ��ɣ�
����⣺��1�����������غ㶨�ɿ�֪�ձ��������ļ�����������������������������������������=100g+10g-109.8g=0.2g��
��2������ҪŨ��������ΪX��X��98%=100g��9.8%�����X=10g���������ˮ��100g-10g=90g��
��3��������������Ϊ100gǡ����ȫ��Ӧ��������0.2g���������Ժ�������100gʱ���ߴﵽ���ֵ���ٸijɺ��ߣ�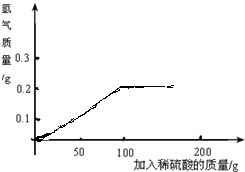
��4����Ҫ������0.2g��������Ҫ�μӷ�Ӧ��п������ΪX����������п������ΪY��
Zn+H2SO4�TZnSO4+H2��
65 161 2
X Y 0.2g
���ݣ�
=
���X=6.5g�����ݣ�
=
���Y=16.1g��
��Ӧ����Һ����Ϊ��6.5g+100g-0.2g=106.3g������������Һ����������������
��100%=15.1%��
�ʴ�Ϊ����1��0.2g����2��90g����3��
��4��15.1%��
��2������ҪŨ��������ΪX��X��98%=100g��9.8%�����X=10g���������ˮ��100g-10g=90g��
��3��������������Ϊ100gǡ����ȫ��Ӧ��������0.2g���������Ժ�������100gʱ���ߴﵽ���ֵ���ٸijɺ��ߣ�

��4����Ҫ������0.2g��������Ҫ�μӷ�Ӧ��п������ΪX����������п������ΪY��
Zn+H2SO4�TZnSO4+H2��
65 161 2
X Y 0.2g
���ݣ�
| 65 |
| 2 |
| X |
| 0.2 |
| 161 |
| 2 |
| Y |
| 0.2 |
��Ӧ����Һ����Ϊ��6.5g+100g-0.2g=106.3g������������Һ����������������
| 16.1g |
| 106.3g |
�ʴ�Ϊ����1��0.2g����2��90g����3��

��4��15.1%��
������������Ҫ���黯ѧ����ʽ����д���йػ�ѧ����ʽ�ļ��㣬�ѶȽ�С����Ҫ�����֪���ձ��������ļ�������������������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ