��Ŀ����
23���ݹ�����������ͳ�ƣ�ȥ��1��10�·ݣ�ȫ������������11��������1123�ˣ�ֱ�ӲƲ���ʧ12.5��Ԫ���ɼ�ÿ���˶�Ӧ���˽�����֪ʶ����ǿ������ȫ��ʶ��
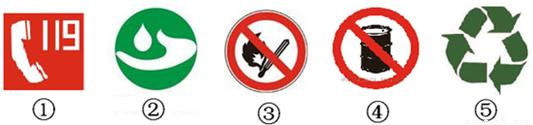
��1�����б�־��������ȫ�йص���

��2���ڼ���ʹ����Ȼ��ʱ��Ϊ��ȫ�����Ӧ�ڳ�����װ��Ȼ��й©����������������װλ����ȷ����ͼ
��3���˽�������������ȷѡ���ʹ���������1211������Ҫ�ɷֵĻ�ѧʽΪCF2ClBr���������������������ǰ�̼������F�����ȡ��壨Br������Ԫ�ص�ԭ�Ӹ������еģ�0��ʾ������������в�����Ԫ�أ�����CF3Br����Ϊ1301���Ʋ�1202�����Ļ�ѧʽΪ
��д��һ������ԭ����
��1�����б�־��������ȫ�йص���
�٢ۢ�
��������ţ�
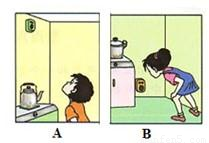
��2���ڼ���ʹ����Ȼ��ʱ��Ϊ��ȫ�����Ӧ�ڳ�����װ��Ȼ��й©����������������װλ����ȷ����ͼ
A
����A��B���������������ʾ©��������Ӧ����ʩ���е����٢�
������ţ��� �ٹر���Դ���š��ڴ��Ŵ��������������̻���3���˽�������������ȷѡ���ʹ���������1211������Ҫ�ɷֵĻ�ѧʽΪCF2ClBr���������������������ǰ�̼������F�����ȡ��壨Br������Ԫ�ص�ԭ�Ӹ������еģ�0��ʾ������������в�����Ԫ�أ�����CF3Br����Ϊ1301���Ʋ�1202�����Ļ�ѧʽΪ
CF2Br2
����ĭ������еķ��ݼ�ͨ������������̼��������Ϊ���ݼ������ߵ�ˮ��Һ�Ӵ������������������������ƺʹ���������̼���ݣ��÷�Ӧ�Ļ�ѧ����ʽΪAl2��SO4��3+6NaHCO3=2Al��OH��3��+3Na2SO4+6CO2��
����д��һ������ԭ����
�����¶ȵ���ȼ����Ż�����£�������������������
����������1������ÿ�ֱ�־�ĺ�����з������
��2��������Ȼ������Ҫ�ɷֵ��ص��й©����������
��3��������������������д����ѧʽ�����ݷ�Ӧ���������д������ʽ���������ԭ���������ɣ�
��2��������Ȼ������Ҫ�ɷֵ��ص��й©����������
��3��������������������д����ѧʽ�����ݷ�Ӧ���������д������ʽ���������ԭ���������ɣ�
����⣺��1���ڢٸ���־�ǻ绰���ڢ۸���־�ǽ�ֹ���̣��ڢܸ���־�ǽ�ֹ����ȼ���־������������ȫ�йأ�
��2����Ȼ������Ҫ�ɷ��Ǽ����ܶȱȿ���С��й©��ۼ����Ϸ�������װ��Ȼ��й©������Ӧ�÷����Ϸ�����Ȼ��й©��Ӧ���ȹرշ��ţ��ٴ��Ŵ�ͨ�磬���ܿ��ƻ����ȣ�
��3�����������������ǰ�̼������F�����ȡ��壨Br������Ԫ�ص�ԭ�Ӹ������еģ����־��Ǹ�Ԫ�����½ǵ����֣�����Ϊ��˵��û�и�Ԫ�أ�����1202�����Ļ�ѧʽΪCF2Br2�����������֪��Ӧ��Ϊ��������̼�����ƣ�������Ϊ���������������ƺͶ�����̼��д����Ӧ�Ļ�ѧ����ʽ���ɣ����ԭ�����ǽ����¶ȵ���ȼ����Ż�����£������������������ȣ�
�ʴ�Ϊ����1���٢ۢܣ���2��A���٢ڣ���3��CF2Br2��Al2��SO4��3+6NaHCO3=2Al��OH��3��+3Na2SO4+6CO2���������¶ȵ���ȼ����Ż�����£������������������ȣ�
��2����Ȼ������Ҫ�ɷ��Ǽ����ܶȱȿ���С��й©��ۼ����Ϸ�������װ��Ȼ��й©������Ӧ�÷����Ϸ�����Ȼ��й©��Ӧ���ȹرշ��ţ��ٴ��Ŵ�ͨ�磬���ܿ��ƻ����ȣ�
��3�����������������ǰ�̼������F�����ȡ��壨Br������Ԫ�ص�ԭ�Ӹ������еģ����־��Ǹ�Ԫ�����½ǵ����֣�����Ϊ��˵��û�и�Ԫ�أ�����1202�����Ļ�ѧʽΪCF2Br2�����������֪��Ӧ��Ϊ��������̼�����ƣ�������Ϊ���������������ƺͶ�����̼��д����Ӧ�Ļ�ѧ����ʽ���ɣ����ԭ�����ǽ����¶ȵ���ȼ����Ż�����£������������������ȣ�
�ʴ�Ϊ����1���٢ۢܣ���2��A���٢ڣ���3��CF2Br2��Al2��SO4��3+6NaHCO3=2Al��OH��3��+3Na2SO4+6CO2���������¶ȵ���ȼ����Ż�����£������������������ȣ�
������������Ҫ����������ȫ��־������Ϣ�Ļ�ȡ���������������������ѧ֪ʶ���н����������

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ