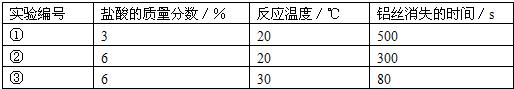
��Ŀ����
�á�����������������=�������к�������д��
��1�������ӵĵ�����ΪX1����ԭ�ӵĵ�����ΪX2��X1
��2��ʢʯ��ˮ��С�ձ�������ΪW1g���ڿ�����¶��һ��ʱ����������ΪW2g��W1
��3������ͭ��Һ������ΪM1g������һ����Ƭһ��ʱ���ȡ����Ƭ��������Һ������ΪM2g��M1
��4����һ�ܱ�������ͨ��һ������һ����̼����������̼ԭ��������ԭ����֮��ΪY1���õ��ȼʹ���ַ�Ӧ����Ӧ��������̼ԭ�Ӻ���ԭ����֮��ΪY2��Y1
��1�������ӵĵ�����ΪX1����ԭ�ӵĵ�����ΪX2��X1
��
��
X2����2��ʢʯ��ˮ��С�ձ�������ΪW1g���ڿ�����¶��һ��ʱ����������ΪW2g��W1
��
��
W2����3������ͭ��Һ������ΪM1g������һ����Ƭһ��ʱ���ȡ����Ƭ��������Һ������ΪM2g��M1
��
��
M2����4����һ�ܱ�������ͨ��һ������һ����̼����������̼ԭ��������ԭ����֮��ΪY1���õ��ȼʹ���ַ�Ӧ����Ӧ��������̼ԭ�Ӻ���ԭ����֮��ΪY2��Y1
=
=
Y2����������1������ԭ�ӡ����ӵĹ�ϵ���Ƚ�ԭ�ӡ����ӵĺ���������Ĵ�С��
��2�����������غ㶨�ɣ��Ƚ�ʢ��ʯ��ˮ��С�ձ�¶�ú������仯��
��3�����������غ㶨�ɣ��Ƚ�����ͭ��Һ������Ƭǰ����Һ�����ı仯��
��4�����������غ㶨�ɵ�Ӧ�÷������
��2�����������غ㶨�ɣ��Ƚ�ʢ��ʯ��ˮ��С�ձ�¶�ú������仯��
��3�����������غ㶨�ɣ��Ƚ�����ͭ��Һ������Ƭǰ����Һ�����ı仯��
��4�����������غ㶨�ɵ�Ӧ�÷������
����⣺��1����ԭ���������1�����ӣ����ʧȥ�����ĵ��ӱ�������ӣ�������ԭ�Ӻ��������X2���������Ӻ��������X1��
��2��ʢʯ��ˮ��С�ձ��ڿ�����¶��ʱ�������տ����еĶ�����̼���γɰ���ɫ����������¶��һ��ʱ���С�ձ�������W2Ҫ����ԭ��С�ձ�������W1��
��3�����������غ㶨�ɣ���Ӧǰ����ͭ��Һ�����������������͵��ڷ�Ӧ����������������Һ�������û���ͭ�������ͣ����ݷ�Ӧ�Ļ�ѧ����ʽFe+CuSO4=Cu+FeSO4��ÿ56�������������û���64��������ͭ������Ӧǰ��������С�ڷ�Ӧ��õ�ͭ����������Ӧ��������Һ������M2С�ڷ�Ӧǰ����ͭ��Һ������M1��
��4�����������غ㶨�ɿ�֪��ѧ��Ӧǰ���ԭ�ӵĸ������ᷢ���仯�����Է�Ӧǰ��̼������ԭ�ӵĸ����Ȳ��䣻
�ʴ�Ϊ����1��������2��������3��������4��=��
��2��ʢʯ��ˮ��С�ձ��ڿ�����¶��ʱ�������տ����еĶ�����̼���γɰ���ɫ����������¶��һ��ʱ���С�ձ�������W2Ҫ����ԭ��С�ձ�������W1��
��3�����������غ㶨�ɣ���Ӧǰ����ͭ��Һ�����������������͵��ڷ�Ӧ����������������Һ�������û���ͭ�������ͣ����ݷ�Ӧ�Ļ�ѧ����ʽFe+CuSO4=Cu+FeSO4��ÿ56�������������û���64��������ͭ������Ӧǰ��������С�ڷ�Ӧ��õ�ͭ����������Ӧ��������Һ������M2С�ڷ�Ӧǰ����ͭ��Һ������M1��
��4�����������غ㶨�ɿ�֪��ѧ��Ӧǰ���ԭ�ӵĸ������ᷢ���仯�����Է�Ӧǰ��̼������ԭ�ӵĸ����Ȳ��䣻
�ʴ�Ϊ����1��������2��������3��������4��=��
�����������漰��֪ʶ��϶࣬��ҪĿ����ͨ����ֵ��С�ıȽϣ�������ѧ�����û�ѧ֪ʶ���������������

��ϰ��ϵ�д�
�����Ŀ