��Ŀ����
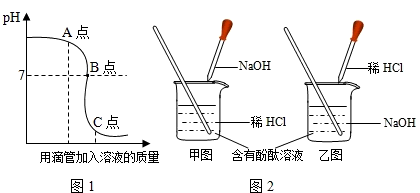
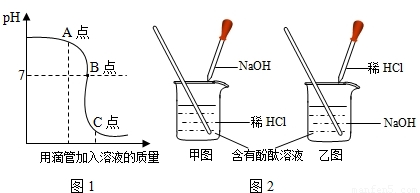
�кͷ�Ӧ��ҽҩ������ũҵ�ϲ������Ź㷺��Ӧ�ã�ͼ1��ʾ�����NaOH��Һ������Ӧ��������Һ��pH�������Һ��������ϵ���������ͼ1�л�ȡ��Ϣ���ش��������⣺
��1��pH��ֽ��ʹ�÷�����
��2��ʵ������ǰ�������
��3��ͼ��A��ʱ����Һ�����ʵĻ�ѧʽΪ
��4����֪100g ������������Ϊ7.3%��ϡ��������100gNaOH��Һǡ����ȫ��Ӧ����
�ٸ�NaOH��Һ����������������
�ڵ�pH=7ʱ���ձ���δ�������������������Һ���������������Ƕ��٣�����д���淶�Ľ��ⲽ�裩

��1��pH��ֽ��ʹ�÷�����
˺һС��pH��ֽ���ڱ������У��ò�����պȡ��Һ����pH��ֽ�ϣ��������ɫ�����ն���pHֵ
˺һС��pH��ֽ���ڱ������У��ò�����պȡ��Һ����pH��ֽ�ϣ��������ɫ�����ն���pHֵ
����2��ʵ������ǰ�������
��
��
�����ң�ͼ��ʾ���У���3��ͼ��A��ʱ����Һ�����ʵĻ�ѧʽΪ
NaOH��NaCl
NaOH��NaCl
����4����֪100g ������������Ϊ7.3%��ϡ��������100gNaOH��Һǡ����ȫ��Ӧ����
�ٸ�NaOH��Һ����������������
8%
8%
���ڵ�pH=7ʱ���ձ���δ�������������������Һ���������������Ƕ��٣�����д���淶�Ľ��ⲽ�裩
��������1������pH��ֽ��ʹ�÷����ش�
��2����������ͼ1��ʼ��Һ��pH�жϣ�
��3������������������Һ�м����ϡ�������������Һ�����ʣ�
��4��������������ϡ����ǡ����ȫ��Ӧʱ����ҺpH=7����������������ϡ����ķ�Ӧ����������Һ�����ʵ������Գ��������ơ��Ȼ����������Ϳ��Խ����йؼ��㣮
��2����������ͼ1��ʼ��Һ��pH�жϣ�
��3������������������Һ�м����ϡ�������������Һ�����ʣ�
��4��������������ϡ����ǡ����ȫ��Ӧʱ����ҺpH=7����������������ϡ����ķ�Ӧ����������Һ�����ʵ������Գ��������ơ��Ȼ����������Ϳ��Խ����йؼ��㣮
����⣺��1��pH��ֽ��ʹ�÷����ǣ�˺һС��pH��ֽ���ڱ������У��ò�����պȡ��Һ����pH��ֽ�ϣ��������ɫ�����ն���pHֵ��
��2��������ͼ1��ʼ��Һ��pH�Ǵ���7��˵���������������Ƶ���Һ�м���ϡ���ᣮ���ԣ�ʵ������ǰ���������ͼ��ʾ���У�
��3��������ͼ1��֪����ͼ��A��ʱ����Һ��pH����7��˵�������������Ƶ���Һ�м���ϡ����������㣬��Һ�������ɵ�NaCl��δ��Ӧ��NaOH��
��4����100gNaOH��Һ�����ʵ�����Ϊx�������Ȼ��Ƶ�����Ϊy
HCl+NaOH�TH2O+NaCl
36.5 40 58.5
100g��7.3% X y
=
��ã�X=8g
=
��ã�y=11.7g
�ٸ�NaOH��Һ���������������ǣ�
��100%=8%
�ڵ���Һ���������������ǣ�
��100%=5.85%
�ʴ�Ϊ����1��˺һС��pH��ֽ���ڱ������У��ò�����պȡ��Һ����pH��ֽ�ϣ��������ɫ�����ն���pHֵ����2���ң���3��NaOH��NaCl����4����8%����������Һ����������������5.85%��
��2��������ͼ1��ʼ��Һ��pH�Ǵ���7��˵���������������Ƶ���Һ�м���ϡ���ᣮ���ԣ�ʵ������ǰ���������ͼ��ʾ���У�
��3��������ͼ1��֪����ͼ��A��ʱ����Һ��pH����7��˵�������������Ƶ���Һ�м���ϡ����������㣬��Һ�������ɵ�NaCl��δ��Ӧ��NaOH��
��4����100gNaOH��Һ�����ʵ�����Ϊx�������Ȼ��Ƶ�����Ϊy
HCl+NaOH�TH2O+NaCl
36.5 40 58.5
100g��7.3% X y
| 36.5 |
| 40 |
| 100g��7.3% |
| X |
| 36.5 |
| 58.5 |
| 100g��7.3% |
| y |
�ٸ�NaOH��Һ���������������ǣ�
| 8g |
| 100g |
�ڵ���Һ���������������ǣ�
| 11.7g |
| 100g+100g |
�ʴ�Ϊ����1��˺һС��pH��ֽ���ڱ������У��ò�����պȡ��Һ����pH��ֽ�ϣ��������ɫ�����ն���pHֵ����2���ң���3��NaOH��NaCl����4����8%����������Һ����������������5.85%��
������������ͼ�����ʽ����������кͷ�Ӧʱ��ҺPH�ı仯�Լ����ʼ��������ϵ����ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ