��Ŀ����
��2011?��ƽ��ģ�⣩ʵ������һƿ���ʵı�ǩ�����䣬ֻ֪������NH4Cl����NH4��2SO4������[CO��NH2��2]�е�һ�֣������������̽��
�����롿
����ٸû�����NH4Cl��
����ڣ��û����ǣ�NH4��2SO4��
����۸û��������أ�
��ʵ��̽����
��1��ȡ������Ʒ���в�������ʯ����ĥ���д̼�����ζ������ų�֤������
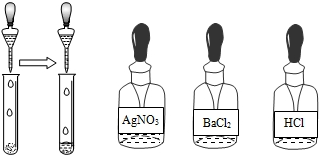
��2����ͼ��ʾ��ȡ������Ʒ���Թ��У��μ�����ˮ���ٵ����е�
�����롿
����ٸû�����NH4Cl��
����ڣ��û����ǣ�NH4��2SO4��
����۸û��������أ�
��ʵ��̽����
��1��ȡ������Ʒ���в�������ʯ����ĥ���д̼�����ζ������ų�֤������
��
��
����������2����ͼ��ʾ��ȡ������Ʒ���Թ��У��μ�����ˮ���ٵ����е�
�Ȼ���
�Ȼ���
��Һ����û�г�������
û�г�������
�������ٳ��������а�ɫ��������
�а�ɫ��������
�������ڳ������䷴Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl
��NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl
��
��������1�����������Ӧ�����д̼�����ζ�İ��������ʷ�����
��2����������������������ӵļ��鷽��������
��2����������������������ӵļ��鷽��������
����⣺��1��������Ʒ���в�������ʯ����ĥ���д̼�����ζ������ų�֤������۲�������
��2������������������뱵���ӽ�����ɳ��������ԣ�ȡ������Ʒ���Թ��У��μ�����ˮ���ٵ����е��Ȼ�����Һ����û�г������ɣ������ٳ��������а�ɫ�������ɣ������ڳ������䷴Ӧ�Ļ�ѧ����ʽΪ����NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl��
�ʴ�Ϊ����1���ۣ���2���Ȼ�����û�г������ɣ��а�ɫ�������ɣ���NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl��
��2������������������뱵���ӽ�����ɳ��������ԣ�ȡ������Ʒ���Թ��У��μ�����ˮ���ٵ����е��Ȼ�����Һ����û�г������ɣ������ٳ��������а�ɫ�������ɣ������ڳ������䷴Ӧ�Ļ�ѧ����ʽΪ����NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl��
�ʴ�Ϊ����1���ۣ���2���Ȼ�����û�г������ɣ��а�ɫ�������ɣ���NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl��
�����������Ĺؼ�������笠����ӡ���������ӵļ��鷽�����ܹ���ȷ��д����Ӧ�ķ���ʽ��

��ϰ��ϵ�д�
�����Ŀ
 ��2011?��ƽ����ģ���۲����»�ѧ��Ӧ��ʾ��ͼ������˵������ȷ���ǣ�������
��2011?��ƽ����ģ���۲����»�ѧ��Ӧ��ʾ��ͼ������˵������ȷ���ǣ�������
