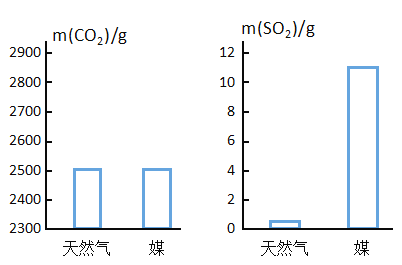
��Ŀ����
����Ŀ�����Ա�ʵ�����ǿ�ѧ�о����õķ���������������ʾ��ʵ��ʾ��ͼ�ش����⣺
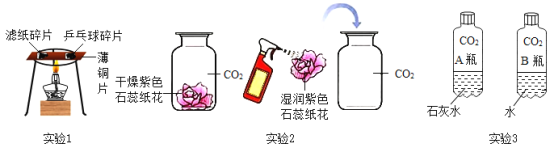
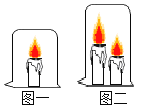
��1��ʵ��1�У�����С��ͬ����ֽƬ��ƹ������Ƭ���ڱ�ͭƬ�����࣬����ͭƬ���в����۲쵽ƹ������Ƭ��ȼ�գ�˵��ƹ������Ƭ���Ż�����ֽƬ_______������������������������ʵ��Ŀ����_____________��
��2��ʵ��2�У�ʵ����Ա�����___________���Ѹ������ɫʯ��ֽ����ʪ�����ɫʯ��ֽ���ֱ������ƿ����Ķ�����̼�����У��۲쵽������________________��ԭ����_________________���û�ѧ����ʽ��ʾ����
��3��ʵ��3�У���2����Ȫˮ����ƿ���۲쵽����ƿ���ij̶�ΪA_________B����������������һ��������С�����������б���ǵ�ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ_________���Աȴ�ʵ������֤��CO2����Ca��OH��2������Ӧ��
���𰸡��� ֤��ȼ��ʱ�¶���Ҫ�ﵽ�Ż�� ˮ ʪ�����ɫʯ��ֽ����� CO2+H2O�TH2CO3 ���� Ca��OH��2+CO2=CaCO3��+H2O
��������
��1��ʵ��1�У�����С��ͬ����ֽƬ��ƹ������Ƭ���ڱ�ͭƬ�����࣬����ͭƬ���в����۲쵽ƹ������Ƭ��ȼ�գ�˵��ƹ������Ƭ���Ż�����ֽƬ�ͣ�ͨ��������˵��ȼ�յ�����֮һ���ǣ��¶ȴﵽ�Ż�㣻����ͣ�֤��ȼ��ʱ�¶���Ҫ�ﵽ�Ż�㣻
��2��ʵ��2�У�ʵ����Ա�����ˮ���Ѹ������ɫʯ��ֽ����ʪ�����ɫʯ��ֽ���ֱ������ƿ����Ķ�����̼�����У�CO2��ˮ��Ӧ����̼�ᣬʪ�����ɫʯ��ֽ����죻
���ˮ��ʪ�����ɫʯ��ֽ����죻CO2+H2O�TH2CO3��
��3��ʵ��3�У���2����Ȫˮ����ƿ���۲쵽����ƿ���ij̶�ΪA����B�����б���ǵ�ƿ�ڷ����ķ�Ӧ�ǣ�������̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��
������ڣ�Ca��OH��2+CO2=CaCO3��+H2O��

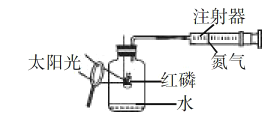
����Ŀ��ijУ��ѧ��ȤС���ͬѧ���������ͼ��ʾ��װ�ý��п�������������ʵ���̽��:
ʵ��ǰע��������20 mL����,100 mL����ƿ�м���10 mLˮ��ʵ�����ݼ�¼����:
ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
ע��������/mL | 20.0 | 34.0 | 40.0 | 30.0 | 25.6 | 18.0 | 12.5 | 8.6 | 4.8 | 2.0 | 2.0 |
��1����������ʵ����̺������ۺϷ���,����ƿ�ڿ����������������____________mL;��ʼ��������ԭ����_______________��
��2����ʽ����������������������______________��
��3�����������ƫС,ԭ�������________________��