题目内容
1909年化学家哈伯在实验室首次合成了氨.2007年化学家格哈德?埃特尔在哈伯研究所证实了氢气与氮气在固体催化剂表面合成氨的反应过程,获得诺贝尔化学奖.(1)将氢气和氮气在高温、高压和催化剂的条件下合成氨(NH3).反应的化学方程式为
(2)用
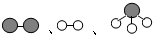 分别表示N2、H2、NH3.观察下图,写出符合在催化剂表面合成氨反应过程的顺序
分别表示N2、H2、NH3.观察下图,写出符合在催化剂表面合成氨反应过程的顺序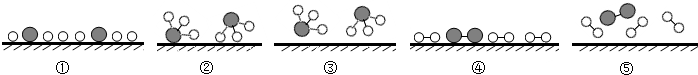
(3)表面化学对于化学工业很重要,它可以帮助我们了解不同的过程.在潮湿的空气里,铁表面吸附了一层薄薄的水膜,同时由于空气中O2的溶解,水膜呈中性.若空气中CO2溶解,水膜呈酸性,写出该反应的化学方程式
分析:书写化学方程式时,必须知道反应物、生成物和反应条件,必须遵守质量守恒定律的两个原则.
(1)该反应是在高温、高压和催化剂三个条件的共同作用下完成的.
(2)可以从化学反应的实质分析、推导,从而得出本题的答案.
(3)第一空答案的得出,必须结合二氧化碳与水的反应和碳酸的酸碱性.
第二空可以根据质量守恒定律得出生成物是氮气和二氧化碳,然后再根据化学方程式的书写方法得出本题的答案.
(1)该反应是在高温、高压和催化剂三个条件的共同作用下完成的.
(2)可以从化学反应的实质分析、推导,从而得出本题的答案.
(3)第一空答案的得出,必须结合二氧化碳与水的反应和碳酸的酸碱性.
第二空可以根据质量守恒定律得出生成物是氮气和二氧化碳,然后再根据化学方程式的书写方法得出本题的答案.
解答:解:(1)氢气和氮气在高温、高压和催化剂的条件下反应生成氨.
该反应的化学方程式为:N2+3H2
2NH3.
(2)化学反应的实质是分子分成原子,原子重新组合成新的分子.因此应从表示氮气和氢气的图形⑤开始,到分子分成原子的图形④,到原子的重新组合的图形①,到形成新分子的图形②,到新分子脱离催化剂的图形③,从而完成整个反应.
(3)水膜中溶解了二氧化碳后,由于二氧化碳能与水化合生成碳酸,碳酸又具有酸性,因此水膜呈酸性.上述反应的化学方程式为:CO2+H2O=H2CO3.
根据空气的组成成分和质量守恒定律可知:两种生成物是氮气和二氧化碳.
因此一氧化碳和一氧化氮在催化剂的作用下反应生成氮气和二氧化碳.
该反应的化学方程式为:2CO+2NO
N2+2CO2.
故答案为:
(1)N2+3H2
2NH3.
(2)⑤④①②③.
(3)CO2+H2O=H2CO3;2CO+2NO
N2+2CO2.
该反应的化学方程式为:N2+3H2
| ||
| 催化剂 |
(2)化学反应的实质是分子分成原子,原子重新组合成新的分子.因此应从表示氮气和氢气的图形⑤开始,到分子分成原子的图形④,到原子的重新组合的图形①,到形成新分子的图形②,到新分子脱离催化剂的图形③,从而完成整个反应.
(3)水膜中溶解了二氧化碳后,由于二氧化碳能与水化合生成碳酸,碳酸又具有酸性,因此水膜呈酸性.上述反应的化学方程式为:CO2+H2O=H2CO3.
根据空气的组成成分和质量守恒定律可知:两种生成物是氮气和二氧化碳.
因此一氧化碳和一氧化氮在催化剂的作用下反应生成氮气和二氧化碳.
该反应的化学方程式为:2CO+2NO
| ||
故答案为:
(1)N2+3H2
| ||
| 催化剂 |
(2)⑤④①②③.
(3)CO2+H2O=H2CO3;2CO+2NO
| ||
点评:本题主要考查化学方程式的书写和化学反应的实质,同时加强了对化学反应的实质的理解和应用.难度较大.

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
 、
、 、
、 分别表示N2、H2、NH3.观察下图,写出符合在催化剂表面合成氨反应过程的顺序
分别表示N2、H2、NH3.观察下图,写出符合在催化剂表面合成氨反应过程的顺序
 分别表示N2、H2、NH3.观察下图,写出符合在催化剂表面
分别表示N2、H2、NH3.观察下图,写出符合在催化剂表面
 、
、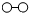 、
、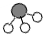 分别表示N2、H2、NH3.在催化剂表面(
分别表示N2、H2、NH3.在催化剂表面( 下图中表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下:
下图中表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下:
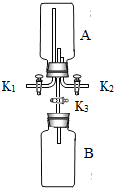 微粒的基本性质”的实验探究,实验过程如下:
微粒的基本性质”的实验探究,实验过程如下:
 、
、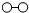 、
、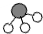 分别表示N2、H2、NH3.在催化剂表面(下图中
分别表示N2、H2、NH3.在催化剂表面(下图中 表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下:
表示催化剂表面)N2和H2反应合成氨的反应过程可用五张图表示如下: