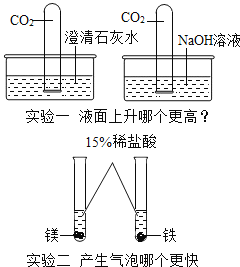
��Ŀ����
����Ŀ��ij�¶��£���ʢ�м�С��KNO3������Թ��У�����10g��10% KNO3�ı�����Һ�ף����̶���ʢ��ˮ���ձ��У���ͼ��ʾ�������ձ��м���ij�����ʺ��裬�Թ��еľ�����ʧ�ˣ��õ�KNO3 ����Һ�ң�
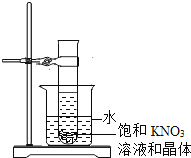
��1����������ʿ����� ������ţ���
a������� b���Ȼ��� c����ʯ�� d����
��2��������Һ���� KNO3����������Ϊ 20%��ԭ�Թ���KNO3���������Ϊ g������Һ ���һ������һ�������DZ�����Һ��
���𰸡���1��C����2��1.25����һ����
����������1�����ձ��м���ij�����ʺ��裬�Թ��еľ�����ʧ�ˣ��õ�KNO3 ����Һ�ң�˵����������ʺ���Һ���¶����ߣ�A�����������ˮ����Һ�¶Ƚ��ͣ�B���Ȼ�������ˮ���¶Ȳ��䣻C����ʯ������ˮ����ˮ��Ӧ���ų��������ȣ��¶����ߣ�D��������ˮ����Һ�¶Ƚ��ͣ���ѡC��
��2��������Һ����KNO3����������Ϊ20%������ԭ�Թ���KNO3���������Ϊx����![]() ��100%=20%�����x=1.25g������Һ����ǡ�ñ��ͣ�Ҳ���ܲ����ͣ��������Һ��һ���DZ�����Һ��
��100%=20%�����x=1.25g������Һ����ǡ�ñ��ͣ�Ҳ���ܲ����ͣ��������Һ��һ���DZ�����Һ��

 �żӾ���ϵ�д�
�żӾ���ϵ�д�����Ŀ����ȥ���������л��е����ʣ���ѡ�õ��Լ���������������ȷ��һ����
ѡ�� | ���� | ���� | ��ȥ�������õ��Լ��Ͳ������� |
A | CuO | Cu | ���� |
B | CO2 | CO | ͨ����������ȼ |
C | Cu(NO3)2��Һ | AgNO3��Һ | ���������ͭ�ۣ����� |
D | ̼��� | �Ȼ��� | ����������ˮ�ܽ⣬���ˡ�ϴ�ӡ����� |