��Ŀ����
�ܽ���ǽ����Һ����������Ҫ���ݡ�
I.�����ܽ�����߽����������: (M��N�������ᾧˮ)
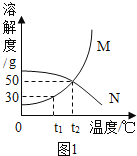
��1��t1��ʱ����20g M����50gˮ�У�����ܽ⣬�γ�_________(����͡������͡�)��Һ����Һ������Ϊ_____________g.�����¶Ȳ��䣬�����Һ���ټ���10gˮ��ֽ��裬��Һ����������������_______(��������С�����䡱) ;
��2��M�����к�������N���ʣ�����__________�����ᴿM����(����½ᾧ���������ᾧ��) ;
��3��t2��ʱ����25gN����50gˮ�У���ȫ�ܽ⡣������߸���Һ���������������������������____________��
II.���ݱ�����ʵ������:
�¶�/��C | 20 | 30 | 50 | 80 | 90 | |
�ܽ��/g | KNO3 | 31.6 | 45.8 | 85.5 | 100 | 169 |
K2CO3 | 110 | 114 | 121 | 126 | 139 |
ijKNO3��Ʒ�к�������K2CO3,���ᴿ������ͼ2(����������ˮ������û�б仯):

��1��ͼ2����ҺC��__________(����͡������͡�)��Һ; .
��2����Ʒ�м�Ԫ�ص�����Ϊ__________g (�����������).
����ʹ����ˮ���ײ��в�ˮ������Ҫ�ɷ�ΪCaCO3��Mg(OH)2��ijѧУ��ѧ�о�С���ͬѧͨ��ʵ��ⶨˮ����CaCO3�ĺ�������������ϡ������뵽200gˮ���У��Ѳ�����CO2������������NaOH��Һ���գ�ͬʱ����3min����NaOH��Һ���ӵ�������������±�:
ʱ��/s | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
����/g | 0 | 30 | 50 | 60 | 66 | 66 | 66 |
(1)д��CO2��������NaOH��Һ����ʱ�������Ļ�ѧ��Ӧ����ʽ_______________��
(2)�ӱ����п��Կ�����200gˮ�������ᷴӦ���ɵ�CO2�����___________g��
(3)����ˮ����̼��Ƶ�����������д���������_________
�ܽ�������dz��л�ѧ�����ĸ����ش��������⡣
I.�±���NaC1��KNO3�ڲ�ͬ�¶��µ��ܽ��:
�¶�/�� | 0 | 20 | 40 | 60 | 80 | |
�ܽ��/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 |
(1)Ҫ�Ƚ�NaC1��KNO3��ˮ�е��ܽ���������Ҫ���Ƶı�����ˮ��������_____________________��
(2)��KNO3��Һ�л�ȡ�侧�����˵ķ�����_____________________��
(3)20��ʱ����ȡNaC1��KNO3����3.5g����10gˮ�У�����ܽ��ﵽ����״̬����___��Һ��
(4)�������������������л��Ƴ�NaCl��KNO3���ܽ�����ߣ��������ߵĽ������Ӧ���¶ȷ�Χ�ǣ�_____��
A 0��C~20��C
B 20��C ~40��C
C 40��C ~60��C
D 60��C ~80��C
II.������Ϊ30g��B���ʼ��뵽50gˮ�У���ֽ����ʣ�����ʵ��������¶ȵĹ�ϵ��ͼ��ʾ�������в�����ˮ���������ش�����:
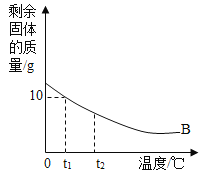
(5)����B���ܽ�����¶ȵ����߶�____________(�����С��) ��
(6) t1��ʱ��B���ܽ����___________��



