��Ŀ����
����Ŀ�������кͷ�Ӧ���Բⶨ������Һ�����ʵ��������������磬��һ�����Ĵ����ᣨ����Һ����μ�����֪���������ļ���ᣩ��Һ������Һ����ֱ���ᡢ��������ȫ��Ӧ���������ı�����ᣩ��Һ�Ķ��ټ�������������ᣨ����Һ������������ ��ͼΪij�βⶨ�����У���Һ��pH�����μӵ�ij����Һ����ı仯���仯�Ĺ�ϵͼ��
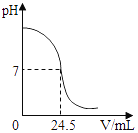
��1��������ͼ���߿����ж�ʵ��������________����������������������ͬ����Һ������Һ���еμ�________��Һ����Һ����
��2������βⶨ�У�Ϊ��ָʾ�кͷ�Ӧ�պ���ȫ���÷�̪��ָʾ�������ڴ�����Һ���ȵ��뼸�η�̪��Һ��������________����֤���кͷ�Ӧ������ɣ�
��3�����ʵ����ʹ�õ��������ᣬ�����������ƣ��Ҵ���Һ�ͱ�Һ���ܶȾ���1.0g/mL�ƣ����ⶨ��ȡ�ô���Һ25mL����Һ���ʵ���������Ϊ4.5%������ͼ�����ݣ��������Һ���ʵ�����������
���𰸡�������Һ�ĺ�ɫ�պ���ȥ3.6%��
��������
��ͼ����Һ��pH�仯�����δ����ʱ��Һ��pH����7���Լ��ԣ�������Һ��pH��С������С��7���ʿ�ʼ�Ǽ�����Һ�������������Һ�еμ�����Һ�������ᡣ
��̪��������Һ���죬��������Һ����ɫ���кͷ�Ӧ�պ����ʱ��Һ�����ԣ���ʱ��ɫ��Ϊ��ɫ���������Һ�ĺ�ɫ�պ���ȥ��
�����Һ���ʵ���������Ϊx��
2NaOH+H2SO4 �TNa2SO4 +2H2O
80 98
25mL��1.0g/mL��x 24.5mL��1.0g/mL��4.5%
![]()
��ã�x=3.6%
�𣺴���Һ���ʵ���������Ϊ3.6%��

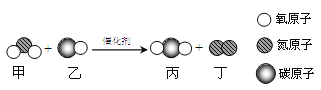
����Ŀ��(1)��ҵ���ü��ҷ�Ӧ�Ʊ�ȼ�ϱ���
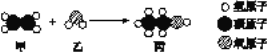
�ٱ���C��H��OԪ�ص�������Ϊ____________��
�ڸ÷�Ӧ�Ļ���������____________________��
(2)�Ƚ����л�ѧ���ﲢ��ɱ������ݡ�
H2O��H2O2��NH3 | ����֮���� |
��__________________________�� | |
��______ | |
| ��֮ͬ���� |
_____________________________�� | |
______________ |
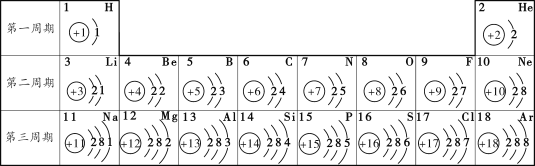
����Ŀ������ͼ������ȷ��ӳ����Ӧ��������
A | B | C | D |

��һ������AgNO3��Һ����μ���NaCl��Һ | ��������Zn��Mg�ֱ�������������������ϡ��������������Ӧ | ��NaOH��Һ����μ���ϡ���� | ��NaOH��Na2CO3�Ļ����Һ�У���μ���ϡ���� |