��Ŀ����
����Ŀ��ʵ������һƿ���Ȼ��غ��������ɵĻ��ʣ�����ע������������ģ�����壬С����ͨ��ʵ�����±�ע�������������е�ʵ�鲽�裺
��ȡ10����Ʒ���ձ��У���������ˮ����ܽ⣬���Ƴ�60����Һ��
�������Ƶ���Һ�У�����һ��������������Һ��ǡ����ȫ��Ӧ��
�����������Һ���ˣ�������ϴ�ӡ�����Ƶ�����Ϊ14.35�ˡ�
���������ʵ��ش�(��ط�Ӧ�Ļ�ѧ����ʽΪ��KCl+AgNO3=AgCl��+KNO3)
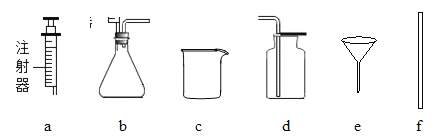
��1���ڹ��˲�����ʹ�õIJ��������У��ձ���______�Ͳ�������
��2����Ʒ���Ȼ��ص���������Ϊ���٣���Ҫ����ʽ���㣩
��3����ȫ��Ӧ���Һ�е�����������Ƕ��ٿˣ�
��4��Ϊ�˻�������أ�С������Һ�����������ᾧ���������������õľ�������С�ڼ���ó�������ֵ��������ֽ����ԭ������� ______�� ��ѡ���ţ�
A������û�и��� B������ʱҺ��ɽ� C������ز����ڲ�������
���𰸡���1��©������2��74.5%����3��12.65g����4��BC
����������1������ʱ��Ҫӵ�µ������У�����̨���ձ���©��������������2�����û�ѧ����ʽ�������Ȼ������Ȼ��������������ȼ��ɼ�����Ȼ��ص�������
�����Ͻ������Ϊx�����ɵ�����ص�����Ϊy��
KCl+AgNO3=AgCl��+KNO3
74.5 143.5 101
X 14.35g y
![]() x=7.45g
x=7.45g
��Ʒ���Ȼ��ص���������=![]() 74.5%��
74.5%��
��3��![]() y=10.1g
y=10.1g
��ȫ��Ӧ���Һ�е����������=10g-7.45g+10.1g=12.65g��
��4��A������û�и���ᵼ������ص�����ƫ����B������ʱҺ��ɽ�����ô�в�������ؽ���������ᵽ�õ�������ص�����ƫС����ȷ��C������ز����ڲ������ϻᵼ�µõ�������ص�����ƫС����ȷ����ѡBC��

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�