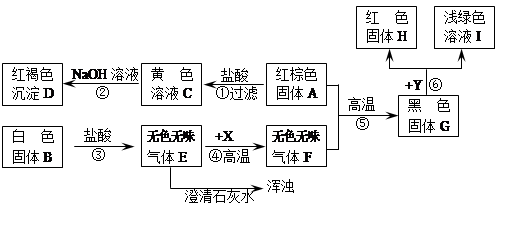题目内容
(6分)A是常见金属单质,B、C是非金属单质,且C是密度最小的气体,甲是磁铁矿的主要成分,它们之间有如下的转化关系(部分产物和反应条件未标明)。
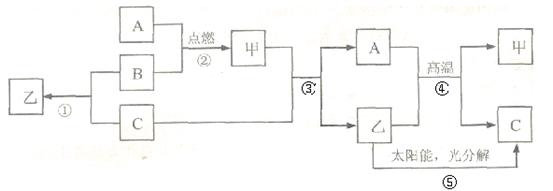
(1)推断甲、乙的化学式为:甲 ,乙 ;
(2)写出反应③的化学方程式 ,该反应体现出物质C的
性,在①②③④⑤五个反应中,属于置换反应的有 个;
(3)反应①除了具有放出热量多,原料不受限制的优点外,还有 的优点。

(1)推断甲、乙的化学式为:甲 ,乙 ;
(2)写出反应③的化学方程式 ,该反应体现出物质C的
性,在①②③④⑤五个反应中,属于置换反应的有 个;
(3)反应①除了具有放出热量多,原料不受限制的优点外,还有 的优点。
(1) Fe3O4 H2O (2) 4H2 + Fe3O4Δ 3Fe+4H2O 还原 2 (3)无污染
(1)磁铁矿的主要成分是四氧化三铁,铁在氧气中燃烧能生成四氧化三铁,A、B、甲等物质分别是铁、氧气、四氧化三铁,化学式分别为:H2;Fe3O4;H2O.故填:H2;Fe3O4;H2O.(2)铁和水在高温条件下反应能生成四氧化三铁和氢气,反应的化学方程式为: 4H2 + Fe3O4Δ 3Fe+4H2O,体现出物质C的还原性。
(3)反应①除了具有放出热量多,原料不受限制的优点外,产物无污染.故填:产物无污染.
(3)反应①除了具有放出热量多,原料不受限制的优点外,产物无污染.故填:产物无污染.

练习册系列答案
相关题目