��Ŀ����
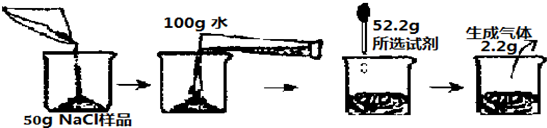
��һ�λ�ѧ�����У���ѧ��ʦ��С��ͬѧ50g NaCl������Ʒ����������Na2CO3���ʣ���Ҫ�������ṩ���Լ���һ����������������������Һ��ϡ���ᡢϡ�����100g��ͨ��ʵ�齫Na2CO3��ȥ���������������ʣ��������������̼���Ƶ�����������
С��ͬѧͨ��˼�����������й��ܽ�������Ժܿ��������ʦ�������������ķ����ǣ�
��ش�
��1����ͬѧ��ѡ�õ��Լ��� ��
��2����50g NaCl��Ʒ������̼���Ƶ����������Ƕ��٣���Ҫ��д��������̣�
��3����ǡ����ȫ��Ӧʱ��������Һ�������Ƕ��٣���Ҫ��д��������̣�
С��ͬѧͨ��˼�����������й��ܽ�������Ժܿ��������ʦ�������������ķ����ǣ�

��ش�
��1����ͬѧ��ѡ�õ��Լ���
��2����50g NaCl��Ʒ������̼���Ƶ����������Ƕ��٣���Ҫ��д��������̣�
��3����ǡ����ȫ��Ӧʱ��������Һ�������Ƕ��٣���Ҫ��д��������̣�
��������1����ѡ������Ҫ��̼���Ʒ�����Ӧ�������Ȼ��Ʒ�����Ӧ���ڳ�ȥ̼���Ƶ�ͬʱ�����ܻ����������ʣ��Ҹ��������֪�������壬����ѡ��ϡ���ᣬ������ϡ���ᣮ
��2�����ݶ�����̼���������̼���Ƶ���������������Ȼ�����Ʒ��̼���Ƶ�����������
��3������������Һ������=���ӵ��������ʵ�������-����������������н��
��2�����ݶ�����̼���������̼���Ƶ���������������Ȼ�����Ʒ��̼���Ƶ�����������
��3������������Һ������=���ӵ��������ʵ�������-����������������н��
����⣺��1����ѡ������Ҫ��̼���Ʒ�����Ӧ�������Ȼ��Ʒ�����Ӧ���ڳ�ȥ̼���Ƶ�ͬʱ�����ܻ����������ʣ��Ҹ��������֪�������壬����ѡ��ϡ���ᣬ������ϡ���ᣬ��Ϊ�����̼���Ʒ�Ӧ���������ƣ������������µ����ʣ����ϡ���
��2����̼���Ƶ�����Ϊx��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
=
x=5.3g
��50gNaCl��Ʒ������̼���Ƶ���������=
��100%=10.6%
��2��������Һ������=50g+100g+52.2g-2.2g=200g
��2����̼���Ƶ�����Ϊx��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
| 106 |
| x |
| 44 |
| 2.2g |
x=5.3g
��50gNaCl��Ʒ������̼���Ƶ���������=
| 5.3g |
| 50g |
��2��������Һ������=50g+100g+52.2g-2.2g=200g
����������һ�������ַ����������ʳ��׳�ȥ������ת��Ϊ�����ʣ���ѡ���ʲ����������ʷ�����Ӧ��

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ