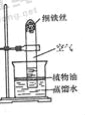
��Ŀ����
����Ŀ��ij��ѧ��ȤС��Ϊ����֤������ˮ���������´���Ӧ�õ��Ļ�����壨���¼�ơ��ϳ��������к������ɵ�������һ����̼��ͬʱ���顰�ϳ��������Ƿ���δ��Ӧ��ļ����ˮ��������������ͼ��ʾ��ʵ�飮
��������֪���������������ڸ��������·�Ӧ��������ˮ��������������ڸ��������·�Ӧ��������������̼��ˮ��
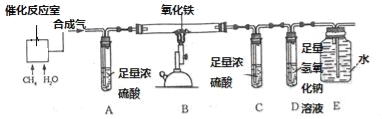
��1����д��������ˮ�����ڸ��´������·�Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ�鿪ʼǰҪ��ͨһ�ᡰ�ϳ�������Ȼ���ٵ�ȼB���ľƾ���ƣ�Ŀ���� ��
��3��װ��C��D������˳��ߵ�����Ӱ��ʵ�������ԭ���� ��
��4��ʵ��������װ��A��C��D�����������ӣ�����װ��C������3.6g��װ��D������3.3g���������ʱ���ϳ�������װ��B�з�Ӧ��ȫ������ƶϳ��á��ϳ��������������Ϊ ��д��ѧʽ����
��5��װ��A��E��ʵ������е����÷ֱ��� �� ��
���𰸡���1��CH4+H2O![]() 3H2+CO����2���ž��������еĿ�������ֹ����ʱ����ը������3������ȷ�ز������ˮ����������4��CO��H2��CH4��CO2����5����ȥˮ�������ռ�β������ֹ��Ⱦ������
3H2+CO����2���ž��������еĿ�������ֹ����ʱ����ը������3������ȷ�ز������ˮ����������4��CO��H2��CH4��CO2����5����ȥˮ�������ռ�β������ֹ��Ⱦ������
����������1���������֪��������ˮ�����ڸ��´������·�Ӧ������һ����̼����������Ӧ�Ļ�ѧ����ʽ Ϊ��CH4+H2O![]() 3H2+CO����2�����ںϳ����ǿ�ȼ�Ե����壬ʵ�鿪ʼǰҪ��ͨһ�ᡰ�ϳ�������Ȼ���ٵ�ȼB���ľƾ���ƣ�Ŀ���ǣ��ž��������еĿ�������ֹ����ʱ����ը����3�������ڸ�ʵ���������Ҫ�ⶨ���ɵ�ˮ������������װ��C��D������˳��ߵ�����Ӱ��ʵ�������ԭ���Dz���ȷ�ز������ˮ����������4����A���������ӣ�˵���˻�������к���ˮ������Cװ�����ӵ����������ɵ�ˮ��������ԭ�����к�����Ԫ�أ�����Ԫ������=3.6g��
3H2+CO����2�����ںϳ����ǿ�ȼ�Ե����壬ʵ�鿪ʼǰҪ��ͨһ�ᡰ�ϳ�������Ȼ���ٵ�ȼB���ľƾ���ƣ�Ŀ���ǣ��ž��������еĿ�������ֹ����ʱ����ը����3�������ڸ�ʵ���������Ҫ�ⶨ���ɵ�ˮ������������װ��C��D������˳��ߵ�����Ӱ��ʵ�������ԭ���Dz���ȷ�ز������ˮ����������4����A���������ӣ�˵���˻�������к���ˮ������Cװ�����ӵ����������ɵ�ˮ��������ԭ�����к�����Ԫ�أ�����Ԫ������=3.6g��![]() ��100%=0.4g��Dװ�����ӵ������Ƿ�Ӧ���ɵĶ�����̼����������̼Ԫ������=3.3g��
��100%=0.4g��Dװ�����ӵ������Ƿ�Ӧ���ɵĶ�����̼����������̼Ԫ������=3.3g��![]() ��100%=0.9g������������C��HԪ��������=12����1��4��=3��1������������C��HԪ��������=0.9g��0.4g=9��4����˿��жϻ�������к��м���ͬʱ����CO��H2���壬���ԣ�����ƶϳ��á��ϳ��������������Ϊ��CO��H2��CH4��CO2����5��װ��A����Ũ���ᣬ������ˮ�֣���ʵ������е������dz�ȥˮ��������Eװ�õ��ص��֪��E�������ǣ��ռ�β����
��100%=0.9g������������C��HԪ��������=12����1��4��=3��1������������C��HԪ��������=0.9g��0.4g=9��4����˿��жϻ�������к��м���ͬʱ����CO��H2���壬���ԣ�����ƶϳ��á��ϳ��������������Ϊ��CO��H2��CH4��CO2����5��װ��A����Ũ���ᣬ������ˮ�֣���ʵ������е������dz�ȥˮ��������Eװ�õ��ص��֪��E�������ǣ��ռ�β����

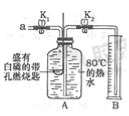
����Ŀ��ijͬѧҪ̽��ȼ�յ������Ϳ����������ĺ���������������ʵ�飮
��ʵ��һ������֪�������Ż��Ϊ40����
ʵ��װ�� | ʵ�鲽�輰���� |
| ������������װ�õ������ԣ����������ã� ����������ʢ�а��Ĵ���ȼ�ճ�����Aƿ�У����ײ�ȼ�գ� ����������Aƿ�м���80����ˮ������ƿ�������ײ�ȼ�գ� ����������K1��K2����a����ƿ�й����������ƿ�е�Һ�����ȼ�ճײ�ʱ���ر�K1��K2����ʱ������ͲB��ˮ�����Ϊ200mL���۲쵽Aƿ�еİ���ȼ�գ� |
��1���ԱȲ����������������֪����ȼ��ȼ�յ�����֮һ��
��2����װ����ȴһ��ʱ���K2������۲쵽 ��˵�����������������Լռ![]() ����ʵ���ÿ������������������С��
����ʵ���ÿ������������������С��![]() ��ԭ������� ����һ�����ɣ���
��ԭ������� ����һ�����ɣ���
��ʵ���������ͼ��ʾװ�ã������������ԭ��Ҳ���ԱȽ�ȷ�ز�������������ĺ�����������װ���е�ϸ��˿����������ϸͭ˿����ʵ�飬�ܷ�Ƚ�ȷ�ز�������������ĺ�������˵������ ������֪��ͭ���������������ˮ��������̼��Ӧ����ͭ�̣���ѧ����ʽ��2Cu+O2+H2O+CO2�TCu��OH��2CO3��