��Ŀ����
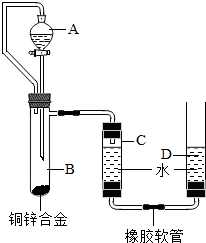 С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ����
С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ����������װ�ò����װ�������ԣ�
�ڼ���ҩƷ��ˮ��
�ۼ�¼C��Һ��̶ȣ�CΪ���п̶ȵIJ����ܣ���
����A��B�еμ��Լ���������
�ݴ�B�в���������������ָ������º�¼C��Һ��̶ȣ�����õ���������ΪV mL�����飬�������ܶ�Ϊ��g/L����
��B��ʣ�������ˡ�ϴ�ӡ����ﲢ���أ��Ƶ�ʣ���������ΪW g��
������������������⣮
��1��B�з�����Ӧ�Ļ�ѧ����ʽΪ
��2����μ����װ�õ�������
��3��С�Ʒ���ʵ��������ȱ��ijһ���赼������ȱ���������㣬�ò�����
��������1��Bװ����п�������ᷴӦ�����Կ�д��д��ѧ����ʽ��
��2�����������ԭ�������ô���ѹǿ��֪ʶ�����ͼ���װ�������Եķ�����
��3����֪���������������ܶȣ���������������������֪��ʣ���������ΪWg������֪��ԭ����������������㣮����ʵ��������ȱ��ijһ���赼������ȱ���������㣬�ò����ǣ�����ͭп�Ͻ����������
��2�����������ԭ�������ô���ѹǿ��֪ʶ�����ͼ���װ�������Եķ�����
��3����֪���������������ܶȣ���������������������֪��ʣ���������ΪWg������֪��ԭ����������������㣮����ʵ��������ȱ��ijһ���赼������ȱ���������㣬�ò����ǣ�����ͭп�Ͻ����������
����⣺��1��ͭп�Ͻ���п�������ᷴӦ��������������п����ͬ������ϡ���ᷴӦ����˸��ݷ�Ӧ������������д��صĻ�ѧ����ʽ��
��2���ӷ�Ӧ��װ���ص��ϴ���ѹǿ��֪ʶ��֪�����װ�õ������Եķ��������ǣ����D�ܣ���ֹƬ�̺�C��DҺ���Դ��ڸ߶Ȳ������������װ�ò�©����
��3����֪���������������ܶȣ���������������������֪��ʣ���������ΪWg������֪��ԭ����������������㣮����ȱ�ٵIJ����ǣ�����ͭп�Ͻ����������
�ʴ�Ϊ����1��Zn+H2SO4=ZnSO4+H2��
��2�����D�ܣ���ֹƬ�̺�C��DҺ���Դ��ڸ߶Ȳ������������
��3������ͭп�Ͻ���Ʒ������
��2���ӷ�Ӧ��װ���ص��ϴ���ѹǿ��֪ʶ��֪�����װ�õ������Եķ��������ǣ����D�ܣ���ֹƬ�̺�C��DҺ���Դ��ڸ߶Ȳ������������װ�ò�©����
��3����֪���������������ܶȣ���������������������֪��ʣ���������ΪWg������֪��ԭ����������������㣮����ȱ�ٵIJ����ǣ�����ͭп�Ͻ����������
�ʴ�Ϊ����1��Zn+H2SO4=ZnSO4+H2��
��2�����D�ܣ���ֹƬ�̺�C��DҺ���Դ��ڸ߶Ȳ������������
��3������ͭп�Ͻ���Ʒ������
������������Ҫ�����˽����Ļ�ѧ���ʺ�һЩʵ�����ע������Լ����ռ����װ�õ������Եķ�������һ���ۺ���ʵ��̽���⣮

��ϰ��ϵ�д�
�����Ŀ
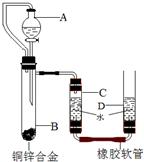
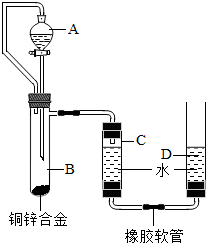 С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ����
С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ���� С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ����������װ�ò����װ�������ԣ��ڼ���ҩƷ��ˮ���ۼ�¼C��Һ��̶ȣ�CΪ���п̶ȵIJ����ܣ�������A��B�еμ��Լ����������ݴ�B�в���������������ָ������º�¼C��Һ��̶ȣ�����õ���������ΪVmL�����飬�������ܶ�Ϊ�� g/L������B��ʣ�������ˡ�ϴ�ӡ����ﲢ���أ��Ƶ�ʣ���������ΪWg��
С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ����������װ�ò����װ�������ԣ��ڼ���ҩƷ��ˮ���ۼ�¼C��Һ��̶ȣ�CΪ���п̶ȵIJ����ܣ�������A��B�еμ��Լ����������ݴ�B�в���������������ָ������º�¼C��Һ��̶ȣ�����õ���������ΪVmL�����飬�������ܶ�Ϊ�� g/L������B��ʣ�������ˡ�ϴ�ӡ����ﲢ���أ��Ƶ�ʣ���������ΪWg��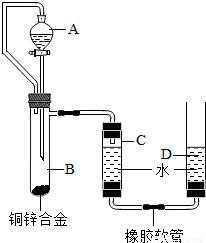 ��2009?���ݣ�С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ����������װ�ò����װ�������ԣ��ڼ���ҩƷ��ˮ���ۼ�¼C��Һ��̶ȣ�CΪ���п̶ȵIJ����ܣ�������A��B�еμ��Լ����������ݴ�B�в���������������ָ������º�¼C��Һ��̶ȣ�����õ���������ΪVmL�����飬�������ܶ�Ϊ�� g/L������B��ʣ�������ˡ�ϴ�ӡ����ﲢ���أ��Ƶ�ʣ���������ΪWg��
��2009?���ݣ�С������ͼװ�òⶨͭп�Ͻ���п������������п�����ԭ����������Ҫʵ�鲽�����£���Ҫ�Ĺ̶�װ������ȥ����������װ�ò����װ�������ԣ��ڼ���ҩƷ��ˮ���ۼ�¼C��Һ��̶ȣ�CΪ���п̶ȵIJ����ܣ�������A��B�еμ��Լ����������ݴ�B�в���������������ָ������º�¼C��Һ��̶ȣ�����õ���������ΪVmL�����飬�������ܶ�Ϊ�� g/L������B��ʣ�������ˡ�ϴ�ӡ����ﲢ���أ��Ƶ�ʣ���������ΪWg��