��Ŀ����
��2013?��ɽ��ģ�����������װ��ͼ�ش����⣺
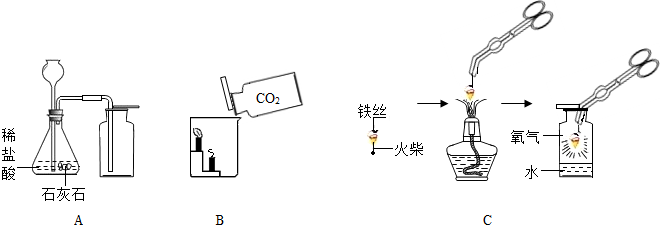
��1��A����ȡ����Ļ�ѧ����ʽΪ
��2��B�У���������̼���������������ȼ��������ձ��У��۲쵽�������ǣ�
��3��С��������˿��������ȼ�յ�ʵ��ʱ��Ϊ�˷�ֹ����ƿ��ը�ѣ���ȷ�IJ�����
��4��С������˿��������ȼ��Ϊʲô������������̽��������þ�Ͳ�ͬ��̼������˿��þ����ֱ����Ϊ0.4mm������������ȼ�գ����������¼���±��У�
������Ϊ����δ���ʵ��������
�������˿��������ȼ��ʱ�����������Ҫԭ����

��1��A����ȡ����Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+CO2��+H2O
CaCO3+2HCl=CaCl2+CO2��+H2O
��Aװ�û�������ʵ������ȡ��һ�����壬��ѧ����ʽΪ2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
��2��B�У���������̼���������������ȼ��������ձ��У��۲쵽�������ǣ�
�²��������Ϩ���ϲ�������Ϩ��
�²��������Ϩ���ϲ�������Ϩ��
��˵��������̼��������ȼ����֧��ȼ�ա��ܶȱȿ�����
����ȼ����֧��ȼ�ա��ܶȱȿ�����
���ʣ���һ������ʵ�������е�Ӧ��Ϊ���
���
����3��С��������˿��������ȼ�յ�ʵ��ʱ��Ϊ�˷�ֹ����ƿ��ը�ѣ���ȷ�IJ�����
��ȼ�ŵ�ϸ��˿���뼯��ƿ����
��ȼ�ŵ�ϸ��˿���뼯��ƿ����
����4��С������˿��������ȼ��Ϊʲô������������̽��������þ�Ͳ�ͬ��̼������˿��þ����ֱ����Ϊ0.4mm������������ȼ�գ����������¼���±��У�
| ���� | þ | ��̼0.05%���� | ��̼0.2%���� | ��̼0.6%���� |
| ȼ��ʱ������ | ����ȼ�գ�����ҫ�⣬���� | ����ȼ�ռ��ٻ��� | ����ȼ���������� | δ�� |
����ȼ�գ���������
����ȼ�գ���������
���������˿��������ȼ��ʱ�����������Ҫԭ����
��˿�к���һ������̼�����
��˿�к���һ������̼�����
����������1��ʯ��ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ݴ���д����ʽ��Aװ�õķ���װ�������ڹ����Һ�岻�������ȡ���壬�����ſ������ռ��ܶȱȿ���������壬ʵ������˫��ˮ��ȡ�������ô�װ�ã�
��2��������̼���ܶȱȿ�������ȼ�գ�Ҳ����֧��ȼ�գ��ݴ˷������������⣻
��3������˿ȼ�յ�ע������������
��4����˿�������о���ȼ�ա��������䣬�����ʵ�������¼�������
��2��������̼���ܶȱȿ�������ȼ�գ�Ҳ����֧��ȼ�գ��ݴ˷������������⣻
��3������˿ȼ�յ�ע������������
��4����˿�������о���ȼ�ա��������䣬�����ʵ�������¼�������
����⣺��1��ʯ��ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O��Aװ�õķ���װ�������ڹ����Һ�岻�������ȡ���壬�����ſ������ռ��ܶȱȿ���������壬ʵ������˫��ˮ��ȡ�������ô�װ�ã���Ӧ�Ļ�ѧ����ʽ�� 2H2O2
2H2O+O2����
��2�����ڶ�����̼���ܶȱȿ�������ȼ�գ�Ҳ����֧��ȼ�գ����Խ�������̼���������������ȼ��������ձ��У��ɹ۲쵽�²��������Ϩ���ϲ�������Ϩ�𣬸��ݶ�����̼����һ�����ص㣬�����г��������
��3��������˿��������ȼ�յ�ʵ��ʱ��Ϊ�˷�ֹ����ƿ��ը�ѣ�Ӧ��ȼ�ŵ�ϸ��˿���뼯��ƿ���룬��Ҫ��������ƿ�ڣ���Ϊ��˿ȼ�����ɵ������������¶Ⱥܸߣ�����������ƿ�ڻ�ʹ����ƿ��ը�ѣ�
��4�����ݼ�¼������̼0.05%�������������о���ȼ�ռ��ٻ��ǣ���̼0.2%����������ȼ���������ǣ��ɴ˿�֪�������˿��������ȼ��ʱ�����������Ҫԭ���ǣ���˿�к���һ������̼����ģ����ź�̼�������ӣ�����Խ���ң��ʺ�̼0.6%�������������о���ȼ�գ��������䣻
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+CO2��+H2O�� 2H2O2
2H2O+O2����
��2���²��������Ϩ���ϲ�������Ϩ�� ����ȼ����֧��ȼ�ա��ܶȱȿ����� ���
��3����ȼ�ŵ�ϸ��˿���뼯��ƿ���룻
��4���پ���ȼ�գ��������䣻 ����˿�к���һ������̼����ģ�
| ||
��2�����ڶ�����̼���ܶȱȿ�������ȼ�գ�Ҳ����֧��ȼ�գ����Խ�������̼���������������ȼ��������ձ��У��ɹ۲쵽�²��������Ϩ���ϲ�������Ϩ�𣬸��ݶ�����̼����һ�����ص㣬�����г��������
��3��������˿��������ȼ�յ�ʵ��ʱ��Ϊ�˷�ֹ����ƿ��ը�ѣ�Ӧ��ȼ�ŵ�ϸ��˿���뼯��ƿ���룬��Ҫ��������ƿ�ڣ���Ϊ��˿ȼ�����ɵ������������¶Ⱥܸߣ�����������ƿ�ڻ�ʹ����ƿ��ը�ѣ�
��4�����ݼ�¼������̼0.05%�������������о���ȼ�ռ��ٻ��ǣ���̼0.2%����������ȼ���������ǣ��ɴ˿�֪�������˿��������ȼ��ʱ�����������Ҫԭ���ǣ���˿�к���һ������̼����ģ����ź�̼�������ӣ�����Խ���ң��ʺ�̼0.6%�������������о���ȼ�գ��������䣻
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+CO2��+H2O�� 2H2O2
| ||
��2���²��������Ϩ���ϲ�������Ϩ�� ����ȼ����֧��ȼ�ա��ܶȱȿ����� ���
��3����ȼ�ŵ�ϸ��˿���뼯��ƿ���룻
��4���پ���ȼ�գ��������䣻 ����˿�к���һ������̼����ģ�
���������⿼���˶�ʵ������ȡ������̼����˿ȼ�յ����֪ʶ��ͬʱ������ѧ����������������������

��ϰ��ϵ�д�
�����Ŀ
