��Ŀ����
����Ŀ����ѧ������ϢϢ��ء�
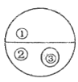
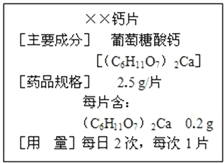
(1)��ͼ��ij��Һƿ��ǩ�ϵIJ�������.�ɴ˿�֪,������������____��Ԫ�����,�����ǵ���Է�������Ϊ_________,Ҫ��10g����������ϡ��Ϊ2%����Һ,��Ҫ��ˮ������Ϊ______g��
(2)������ϵ��й����������ϵ��Ȳ�,�ó����ٴγ�Ϊ����ʩչ���յ���̨���������ʦ�и���������:����ʱ,�ּ��Ͼ��ּӴ�,��ʹ�˱�������ɿ�,ԭ���Ǵ��е��������Ͼ��е��Ҵ����������������±����Ǽ��ֳ�������,�������������:
�������� | ������� | �������� | ������� | �������� |
��ѧʽ | C2H4O2 | C3H6O2 | C3H6O2 | X |
�ټ������(C2H4O2)��̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ_______________��
�ڼ�������(C3H6O2)��̼Ԫ�ص���������Ϊ________(��������ȷ��0.1%)��
�۱ȽϹ�����ѧϰ��ѧ����Ҫ����,�ݱ��Ʋ�X�Ļ�ѧʽΪ____________��
���𰸡�3 180 15 6��1��8 48.6% C4H8O2
��������
��1���������ʵ���ɡ���Է��������ļ��㷽������Һϡ���������ʵ�����������������
��2�����ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȡ�Ԫ�����������ļ��㷽������ѧʽ����д���ɽ��з�����
��1���������ǵĻ�ѧʽ��֪��������̼���⡢��3��Ԫ����ɵģ������ǵ���Է���������12��6+1��12+16��6=180����10g������������Һϡ��Ϊ2%����Һ������Ҫ��ˮ������Ϊx��������Һϡ��ǰ�����ʵ��������䣬��10g��5%=��10g+x����2% ���x=15g��
��2���ټ��������C2H4O2����̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ����12��2������1��4������16��2��=6��1��8��
�ڼ���������C3H6O2����̼Ԫ�ص���������Ϊ![]() =48.6%��
=48.6%��
�۸��ݼ�����������������Ļ�ѧʽ��֪������������һ������������������һ���������1��̼ԭ�ӡ�2����ԭ�ӣ�������������Ļ�ѧʽΪC4H8O2��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������������������Ȼ��Դ�������е����ĺ�����࣬�����ڸ��¡������������¿���ijЩ���ʷ�����Ӧ��ͼ1���Կ�����������Ҫ��ԭ�Ϻϳɵ��ʣ�NH4HCO3�������̣��밴Ҫ��ش��������⣺

��1��������п����õ�����������__________��ͬ���������������������Һ̬�����ɵõ��ϳɰ�����Ҫ�ĵ�������___________�仯������������ߡ���ѧ������
��2��д��������з�����Ӧ�ķ��ű���ʽ___________________________________��
��3��ͼ2�ǻ���̼����藺�װ���ϵIJ���˵����̼����茶��е�������________������ĸ����
A��������ˮ B���лӷ��� C�������ֽ�
��4�����ֻ��ʺ������Ƿ�ﵽ16%���������⣬��ȤС���ͬѧȡ��һЩ������Ʒ������ʵ���ң�
���������ϣ�
�ټ�ʯ���ܹ�����ˮ��CO2�����Dz�����NH3��
��Ũ����������NH3���Dz�����CO2��
�۰�������ˮ�γɰ�ˮ
������̽������ͬѧ��������µ�ʵ��װ�ã�

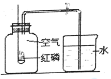
����Aװ�ø�̼����識��ȣ�װҩƷǰ��������е�һ��������_______________��
��ȡ����̼����識����Թܣ�����A��C��Eװ�ã����ȣ�E�е�������_____________��
������A��Bװ�ã��������ȣ��۲쵽�Թܿڵ�������____________֤����ˮ���ɣ�B�е�������______________________________________֤���ж�����̼���ɡ�
��̼�����������ʱ������Ӧ�ķ��ű���ʽ��___________________________________��
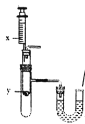
��������������װ��A��C��D�������ӣ�����20g������Ʒ��������A�й�����ȫ��ʧ��
������װ��D�����������
ʵ��ǰDװ�õ����� | 149g |
ʵ���Dװ�õ����� | 152.4g |
���ɴ˷�����֪����Ӧ�в�������������Ϊ__________g��
��ͨ����Ӧ�ı���ʽ����֪���������е�Ԫ��ȫ��������̼����泥����������в�����Ԫ�أ��������˻��ʺ���Ԫ����������Ϊ____________��
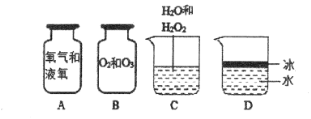
����Ŀ������ʵ����Ʋ��ܴﵽʵ��Ŀ�ĵ��ǣ� ��
ѡ�� | A | B | C | D |
ʵ����� |
|
|
|
|
ʵ��Ŀ�� | ̽����������������� CO2 �����ߵ� | ̽��Ӱ��ľ̿ȼ�վ��ҳ̶ȵ����� | ̽�������Ϩ��ʱ�����İ��̵����� | ̽��Ӱ������˶��������� |
A. A B. B C. C D. D