��Ŀ����
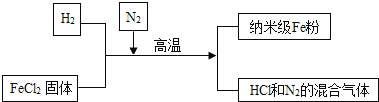
����Ŀ�����Ͳ������� Fe �۾��й㷺����;��������ͨ Fe �۸�����������Ӧ�����Ʊ�����������ͼ��ʾ����ش��������⣺
��1������ Fe �������������Է�ȼ�����ɺ�ɫ���壬�˺�ɫ����Ļ�ѧʽΪ ��
��2���벹����������Ʊ����յĻ�ѧ����ʽ��H2+FeCl2![]() Fe+ ��
Fe+ ��
���Ʊ����� Fe �۵Ĺ��������У�N2�������� ��
��3���о���Ա��������Ƶõ����� Fe ����Ʒ�л���������FeCl2���ʣ�
��Ϊ��ȥ��Ʒ�е����ʣ������������£��ȼ� �ܽ⣬Ȼ���� ��ϴ�ӡ���ɣ�
��ȡ��Ʒ20g���������������ᣬ�������� 0.7g����������Ʒ�е��� Fe ������������
�������������� ��
���𰸡���1��Fe3O4
��2��2HCl ������
��3���� ˮ ���� ��98%��
��������
�����������1������������ȼ���������������������Ժ�ɫ������������������
��2����Ӧ��ķ����к���2����ԭ�ӡ�һ����ԭ�ӡ�2����ԭ�ӣ����������Ѿ�����һ����ԭ�ӣ���ȱ��2����ԭ�Ӻ�2����ԭ�ӣ����Բ����������2HCl�������ڷ�Ӧǰ��û�䣬���ڴ�����������������ýд����ã�
��3������������ˮ���Ȼ���������ˮ�����Լ�ˮ�ܽ⣬�ٽ��й��ˡ�ϴ�Ӹ��T�ɣ�
��������0.7g������Ҫ�μӷ�Ӧ����������ΪX����
Fe+2HCl�TFeCl2+H2��
56 2
X 0.7g
���ݣ�![]() ���X=19.6g��
���X=19.6g��
��������������![]() ��100%=98%
��100%=98%
���ԡ�

 ��У����ϵ�д�
��У����ϵ�д�