��Ŀ����
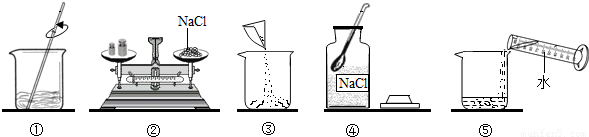
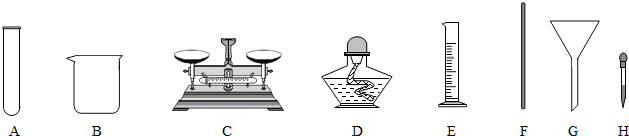
��ͼ��С��ͬѧ����100g 10% NaCl��Һ��ʵ�����ʾ��ͼ��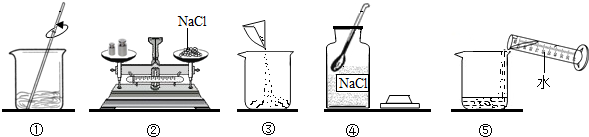
��1����ʵ����ȷ�IJ���˳����
��2������۲� ָ��ͼ�д��� �IJ�������
��3�����Ƹ���Һ��ҪNaCl����
 ��4��С��ͬѧ��������ϵ�ʳ��ת�Ƶ��ձ���ʱ������������ʳ�������������ϣ�������ʹ�����Ƶ���Һ��������������
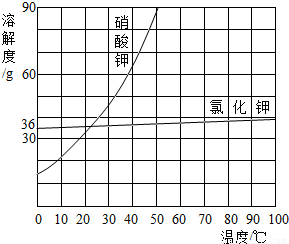
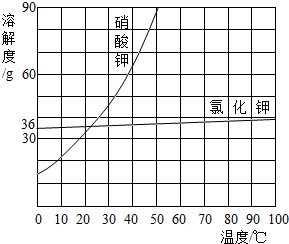
��4��С��ͬѧ��������ϵ�ʳ��ת�Ƶ��ձ���ʱ������������ʳ�������������ϣ�������ʹ�����Ƶ���Һ����������������5�����������ܽ��������ͼ��ʾ����20��ʱ����40g NaCl������뵽100gˮ�У�����ʹ�����ܽ⣬����Ϊ����NaCl��Һ��������
��������1����������������������һ������Һ�Ļ������迼�ǣ���2����������ƽ�������ʵ�ע������ǣ���3����������=��Һ���������������������ܼ�����=��Һ����-������������4�������������������ļ��㷽�����ǣ���5�������ܽ�ȿ��Ǽ���������Ƿ�ȫ���ܽ��ٽ��м��㣮
����⣺��1������������������һ������Һ�Ļ������裺���㡢��������ȡ���ܽ⡢װƿ��ţ�
��2������ʱ�������룬�Ȼ��Ʋ���ֱ�ӷŵ������ϣ�
��3���Ȼ���������100g��10%=10g����ˮ��100g-10g=90g��ˮˮ�����
=90mL��
��4������������ʳ�������������ϣ��������ˣ�����������������С�ˣ�
��5��20��ʱ��NaCl�ܽ��Ϊ36g˵��100gˮ�������36g��������Һ����Ϊ36g+100g=136g��������������Ϊ��
��100%=26.5%
�ʴ�Ϊ����1���ܢڢۢݢ٣�2���ڣ�3��10��90����4������5��136��26.5
��2������ʱ�������룬�Ȼ��Ʋ���ֱ�ӷŵ������ϣ�
��3���Ȼ���������100g��10%=10g����ˮ��100g-10g=90g��ˮˮ�����
| 90g |
| 1g/mL |
��4������������ʳ�������������ϣ��������ˣ�����������������С�ˣ�
��5��20��ʱ��NaCl�ܽ��Ϊ36g˵��100gˮ�������36g��������Һ����Ϊ36g+100g=136g��������������Ϊ��
| 36g |
| 136g |
�ʴ�Ϊ����1���ܢڢۢݢ٣�2���ڣ�3��10��90����4������5��136��26.5
�������������׳����ĵط���Ҫ����ܽ������������������Ƿ�ȫ���ܽ⣬���ܽ�IJ�������Һ������

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ
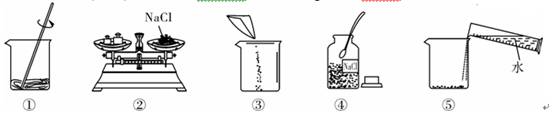

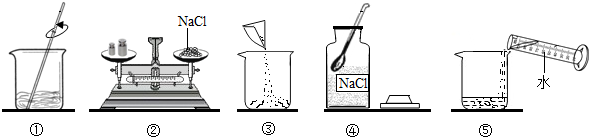
 ��4��С��ͬѧ��������ϵ�ʳ��ת�Ƶ��ձ���ʱ������������ʳ�������������ϣ�������ʹ�����Ƶ���Һ��������������______10%���������=����������
��4��С��ͬѧ��������ϵ�ʳ��ת�Ƶ��ձ���ʱ������������ʳ�������������ϣ�������ʹ�����Ƶ���Һ��������������______10%���������=����������