��Ŀ����
��Դ��������������������ᷢչ������ء�
��1��Ŀǰ����ͨ����ѧ��Ӧ��õ�������������� ��ʯ�͡���Ȼ���Ȼ�ʯȼ�ϡ�
��2����������������� ��������������Ҫ��ɣ�ǰ����Ϊ��̬��Ⱦ����һ����Ǽ�������������Ⱦ��������ס�
�� �����ж���������Դ������������β������ѧʹ�����������ʹ��������
ȼ�ϵ�ԭ���ǣ��û�ѧ����ʽ��ʾ��________��
�� ����������Է���������ˡ����Щ����ά���ص�ʳ��ɴﵽ��Ρ���Ρ�
���εĹ�Ч������ʳ���У�����ά���ص��� ������ĸ��ţ���
|
|
|
|
| A���� | B���߲� | C��ˮ�� |
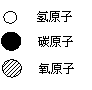 ��3����ҵ���ü��ҷ�Ӧ�Ʊ�ȼ�ϱ���
��3����ҵ���ü��ҷ�Ӧ�Ʊ�ȼ�ϱ���
 | |||||||||
 | |||||||||
 | |||||||||
| |||||||||
| |||||||||
�� ����C��H��OԪ�ص�������Ϊ  ��
��
�� ��Ӧ�мס��ҡ����ķ��Ӹ�����Ϊ ��
��1��ú
|
�� 2H2+O2 2H2O
�� BC
��3��12: 3: 8
��4��1:1:1

��ϰ��ϵ�д�
�����Ŀ


