��Ŀ����
��ͼ��ij���������ռ����Ҫ���̡�

(1)X�Ļ�ѧʽΪ ��
(2)�ó����������������˺�̼���75%��ʯ��ʯ12t�������Ͽ��Ƶ������� t��
(3)������A�봿���ڷ�Ӧ���з�Ӧ�Ļ�ѧ����ʽΪ ��
(4)�ᾧ�õ��Ĺ����ռ���ⶨ�����������Ƶ���������Ϊ99.2%�����������������ʣ������� ��ԭ���� ��
(5)��Һ2�е����ʵĻ�ѧʽΪ ��Ϊ�˽��������ɱ��ͷ�ֹ�Ի��������Ⱦ����Ľ����� ��
(1) H2O (2) 5.04t
(3)Ca(OH)2 + Na2CO3 = CaCO3��+ 2NaOH
(4)Na2CO3 NaOH��Һ�ڹ��ˡ��������ᾧ�����������˿����е�CO2����
(5)Ca(OH)2 ����ҺCa(OH)2����ע�뵽��Ӧ����ѭ������
��������
���������(1)���������غ㶨�ɣ���ѧ��Ӧǰ���Ԫ��ԭ�Ӹ�����Ӧǰ�䣬��X�Ļ�ѧʽΪH2O��(2)�����ķ���ʽΪ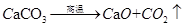 ���μӷ�Ӧ��̼��ƺ������Ƶ�������Ϊ100:56=25:14�����������Ͽ��Ƶ�������
���μӷ�Ӧ��̼��ƺ������Ƶ�������Ϊ100:56=25:14�����������Ͽ��Ƶ�������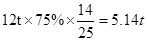 ��(3)AΪ�������ƣ��ʹ�������ֽⷴӦ�������������ƺ�̼��ƣ�����ʽΪ
��(3)AΪ�������ƣ��ʹ�������ֽⷴӦ�������������ƺ�̼��ƣ�����ʽΪ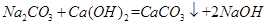 ��(4)���е�������̼���ƣ���ΪNaOH��Һ�ڹ��ˡ��������ᾧ�����������˿����е�CO2���壻(5)�����к���̼��ƺ��������ƣ�ϴ�ӹ��˺���Һ�к����������ƣ�Ϊ�˽��������ɱ��ͷ�ֹ��Ⱦ������Ӧ���������ƻ���ѭ�����á�
��(4)���е�������̼���ƣ���ΪNaOH��Һ�ڹ��ˡ��������ᾧ�����������˿����е�CO2���壻(5)�����к���̼��ƺ��������ƣ�ϴ�ӹ��˺���Һ�к����������ƣ�Ϊ�˽��������ɱ��ͷ�ֹ��Ⱦ������Ӧ���������ƻ���ѭ�����á�
���㣺�����ռ������
���������⿼��֪ʶ���Ϊİ��������ϸ�����֪�����˼��ͻ���֪ʶ�㶼���п�Ҫ��Ļ���֪ʶ��������һ�����Ѷȣ�Ҫ����ᣬ����ϵ��ɡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
