��Ŀ����
ijУ��ȤС��ͬѧΪ̽�����Ļ�ѧ���ʣ���ͼ��������������ʵ�飬��ش��й����⣺

��1��ʵ��һ����������Ӧ�Ļ�ѧ����ʽ�� ��2�֣���
ʵ���������ܲ����IJ�������� ��
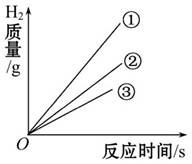
��2��ʵ������ر�Kһ��ʱ��۲쵽������Һ����������K���μ�ϡ���ᣬ�۲쵽������Һ���½������ܿ�������ð�����ر�K��������Һ��������ԭ���� ��������Һ���½���ԭ���� �����û�ѧ����ʽ���ͣ���2�֣�
��3��ʵ������ʵ��۲쵽�������ǣ� ��
��һ����˵�� ���������ͭ�����Ļ�Ը�ǿ��

��1��ʵ��һ����������Ӧ�Ļ�ѧ����ʽ�� ��2�֣���
ʵ���������ܲ����IJ�������� ��
��2��ʵ������ر�Kһ��ʱ��۲쵽������Һ����������K���μ�ϡ���ᣬ�۲쵽������Һ���½������ܿ�������ð�����ر�K��������Һ��������ԭ���� ��������Һ���½���ԭ���� �����û�ѧ����ʽ���ͣ���2�֣�
��3��ʵ������ʵ��۲쵽�������ǣ� ��
��һ����˵�� ���������ͭ�����Ļ�Ը�ǿ��
��1��3Fe+2O2 Fe3O4��2�֣�������ƿ����
Fe3O4��2�֣�������ƿ����
��2��������������ƿ�ڵ�������ƿ����ѹС��������ѹ��Һ��������Fe+2HCl=FeCl2+H2��
��3���к�ɫ������������ɫ��Һ��dz��ɫ����
 Fe3O4��2�֣�������ƿ����
Fe3O4��2�֣�������ƿ������2��������������ƿ�ڵ�������ƿ����ѹС��������ѹ��Һ��������Fe+2HCl=FeCl2+H2��
��3���к�ɫ������������ɫ��Һ��dz��ɫ����
�����������1��ʵ��һ����˿ȼ�յ�ʵ�飬���Է�Ӧ�Ļ�ѧ����ʽ�ǣ�3Fe+2O2
 Fe3O4��ʵ��ǰҪ��ƿ����ƿ�ײ���������ϸɳ���������ˮ����ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը��
Fe3O4��ʵ��ǰҪ��ƿ����ƿ�ײ���������ϸɳ���������ˮ����ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը����2�������п�����ˮ������£������⣬�ر�Kһ��ʱ���������������ƿ�ڵ�������ƿ����ѹС��������ѹ��Һ����������K���μ�ϡ���ᣬ������ϡ���ᷴӦ����������ʹ��ƿ�ڵ��������࣬ѹǿ�������Թ۲쵽������Һ���½��������ķ�Ӧ��ѧ����ʽ��Fe+2HCl=FeCl2+H2��
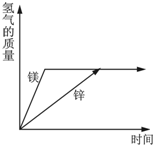
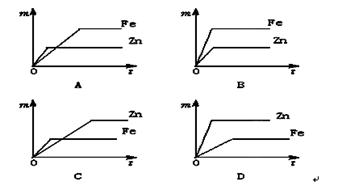
��3���������Ļ�Ա�ͭǿ�����������û�������ͭ��Һ�е�ͭ�����������ǣ��к�ɫ������������ɫ��Һ��dz��ɫ

��ϰ��ϵ�д�
�����Ŀ