��Ŀ����
ͨ���������ϣ�С��ͬѧ����ѧϰС���ͬѧ�˽�������������⣬������Ҳ����������������Һ�ֽ�Ĵ��������������������������Һ�ֽ�Ĵ�����˵��������С�ŵ�̽����Ȥ������С���������µ�̽������1��������������ʵ�鱨�棺
| ʵ����� | ʵ������ | ʵ����� |
| ��һ֧�Թ��м���5ml 6%�Ĺ���������Һ��Ȼ����뼸С�������飬�Ѵ����ǵ�ľ�������Թܿڣ� | ______ | ������ɼӿ����������Һ�ֽ⣬�Ǹ÷�Ӧ�Ĵ����� |
��3���ڸ�ʵ���У��йط�Ӧ�����ֱ���ʽ����ѧ����ʽΪ����______
���𰸡���������1�����ݹ���������Һ�ֽ�ų������Լ�����������ȼ�Խ��н��
��2�����ݴ����ĺ�����н��
��3�����ݹ���������Һ�ڶ�������������������·ֽ�ų��������н��
��4�����ݶ������̵����������Լ��������̲�����ˮ���н��
����⣺��1������������Һ�ֽ�ų�����������������ȼ�ԣ���ʹ�����ǵ�ľ����ȼ������ʵ������Ϊ���Թ��в������������ݣ������ǵ�ľ����ȼ��
��2��������ָ�ܸı仯ѧ��Ӧ���ʣ��������������ͻ�ѧ���ʲ��䣬���Դ�������ӿ����������Һ�ֽ����������˵���������Ǹ÷�Ӧ�Ĵ�������Ϊû����ʵ˵���������ڸ÷�Ӧ��ǰ���������ͻ�ѧ���ʶ����䣻
��3������������Һ�ڶ�������������������·ֽ�ų���������Ӧ�����ֱ���ʽ����ѧ����ʽΪ������������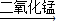 ˮ+������2H2O2
ˮ+������2H2O2 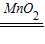 2H2O+O2������
2H2O+O2������
��4���������̵����������У���ɫ���壬������ˮ����Ϊ�������̲�����ˮ�����Կ��ù��˵ķ����ӱ�ʵ��ķ������л������ã�
�ʴ�Ϊ����1���Թ��в������������ݣ������ǵ�ľ����ȼ����2����������ӿ����������Һ�ֽ����������˵���������Ǹ÷�Ӧ�Ĵ�������Ϊû����ʵ˵���������ڸ÷�Ӧ��ǰ���������ͻ�ѧ���ʶ����䣻
��3����������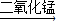 ˮ+������2H2O2
ˮ+������2H2O2 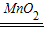 2H2O+O2������
2H2O+O2������
��4����ɫ�����壻������ˮ�����ˣ�
������������Ҫ������������ʵ�������ط������֤����Ľ�ʵ�鷽���ȷ����֪ʶ���ѶȽϴ�
��2�����ݴ����ĺ�����н��
��3�����ݹ���������Һ�ڶ�������������������·ֽ�ų��������н��
��4�����ݶ������̵����������Լ��������̲�����ˮ���н��
����⣺��1������������Һ�ֽ�ų�����������������ȼ�ԣ���ʹ�����ǵ�ľ����ȼ������ʵ������Ϊ���Թ��в������������ݣ������ǵ�ľ����ȼ��
��2��������ָ�ܸı仯ѧ��Ӧ���ʣ��������������ͻ�ѧ���ʲ��䣬���Դ�������ӿ����������Һ�ֽ����������˵���������Ǹ÷�Ӧ�Ĵ�������Ϊû����ʵ˵���������ڸ÷�Ӧ��ǰ���������ͻ�ѧ���ʶ����䣻
��3������������Һ�ڶ�������������������·ֽ�ų���������Ӧ�����ֱ���ʽ����ѧ����ʽΪ������������
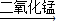 ˮ+������2H2O2
ˮ+������2H2O2 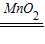 2H2O+O2������
2H2O+O2��������4���������̵����������У���ɫ���壬������ˮ����Ϊ�������̲�����ˮ�����Կ��ù��˵ķ����ӱ�ʵ��ķ������л������ã�
�ʴ�Ϊ����1���Թ��в������������ݣ������ǵ�ľ����ȼ����2����������ӿ����������Һ�ֽ����������˵���������Ǹ÷�Ӧ�Ĵ�������Ϊû����ʵ˵���������ڸ÷�Ӧ��ǰ���������ͻ�ѧ���ʶ����䣻
��3����������
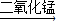 ˮ+������2H2O2
ˮ+������2H2O2 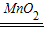 2H2O+O2������
2H2O+O2��������4����ɫ�����壻������ˮ�����ˣ�
������������Ҫ������������ʵ�������ط������֤����Ľ�ʵ�鷽���ȷ����֪ʶ���ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
ͨ���������ϣ�С��ͬѧ����ѧϰС���ͬѧ�˽�������������⣬������Ҳ����������������Һ�ֽ�Ĵ��������������������������Һ�ֽ�Ĵ�����˵��������С�ŵ�̽����Ȥ������С���������µ�̽����
��1��������������ʵ�鱨�棺
| ��������������ʵ����� | ʵ������ | ʵ����� |
| ��һ֧�Թ��м���5ml 6%�Ĺ���������Һ��Ȼ����뼸С�������飬�Ѵ����ǵ�ľ�������Թܿڣ� | ________ | ������ɼӿ����������Һ�ֽ⣬�Ǹ÷�Ӧ�Ĵ����� |
��3���ڸ�ʵ���У��йط�Ӧ�����ֱ���ʽ����ѧ����ʽΪ����________��
��4�����ʹ�ö�������������������Һ�ֽ�Ĵ�������ͨ��ʵ����ʵ��֪���������̾��е�����������________��________��________������________�����ӱ�ʵ��ķ������л������ã�
ͨ���������ϣ�С��ͬѧ����ѧϰС���ͬѧ�˽�������������⣬������Ҳ����������������Һ�ֽ�Ĵ��������������������������Һ�ֽ�Ĵ�����˵��������С�ŵ�̽����Ȥ������С���������µ�̽����
��1��������������ʵ�鱨�棺
��2��ѧϰС��ͬѧ������ʱ��С��ͬѧ���õ�ʵ����۲������ɣ������������ۣ���Ŀ�����______��
��3���ڸ�ʵ���У��йط�Ӧ�����ֱ���ʽ����ѧ����ʽΪ����______
��1��������������ʵ�鱨�棺
| ʵ����� | ʵ������ | ʵ����� |
| ��һ֧�Թ��м���5ml 6%�Ĺ���������Һ��Ȼ����뼸С�������飬�Ѵ����ǵ�ľ�������Թܿڣ� | ______ | ������ɼӿ����������Һ�ֽ⣬�Ǹ÷�Ӧ�Ĵ����� |
��3���ڸ�ʵ���У��йط�Ӧ�����ֱ���ʽ����ѧ����ʽΪ����______