��Ŀ����
����Ŀ�������dz��л�ѧ�г�����ʵ��װ��ͼ���밴Ҫ��������⡣
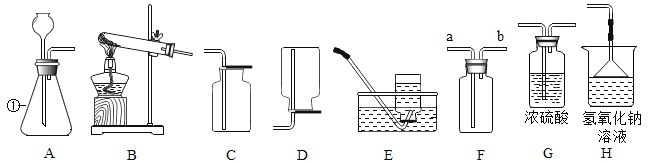
��1������������������______________��
��2����Bװ����Eװ����ȡ��������Ļ�ѧ����ʽΪ____________________��
��3����װ��A��D������ȡ����Ļ�ѧ����ʽ________________��
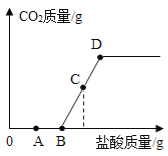
��4���������Ȼ��������ˮ��Һ���������������������ˮ��Һ��ʵ��������������FeS����������ᰴ���ֽⷴӦ��ȡ�������壬����ȡװ������ȡ������̼��ͬ�������ɵ����������ж����ŷ��ڿ����л���Ⱦ������
����ȡ��������Ļ�ѧ����ʽΪ____________��
���������������װ��H���գ��䷴Ӧ�Ļ�ѧ����ʽΪ_________��©����������__________��
���𰸡���1����ƿ
��2��2KClO3 ![]() 2KCl +3O2��
2KCl +3O2��
��3�� Zn+ 2H2SO4==ZnSO4+ H2����Zn+ 2HCl==ZnCl2+ H2��
��4����FeS+ 2HCl==FeCl2+ H2S����H2S + 2NaOH == Na2S + 2H2O ������
��������
�����������1������������ʶ�ǣ���ƿ
��2�����巢��װ�õ�ѡ�����ݣ���Ӧ���״̬�ͷ�Ӧ������Bװ�������ڹ�������ڼ��������·�Ӧ�����Ǽ�������غͶ���������ȡ��������ѧ����ʽΪ��2KClO3 ![]() 2KCl +3O2��
2KCl +3O2��
��3��װ��A�����ڹ�Һ���������ռ�װ�õ�ѡ��������������ܶȺ��ܽ�����ѡ��Eװ���ռ�������˵���������ܶȱȿ���С��������������װ��A��D������ȡ����Ļ�ѧ����ʽΪ��Zn+ 2H2SO4==ZnSO4+ H2����Zn+ 2HCl==ZnCl2+ H2��
��4����ʵ��������������FeS����������ᰴ���ֽⷴӦ��ȡ�������������ո��ֽⷴӦ���ص���������ɷ������������ֻ��������ʿ��ж���һ�ֻ�����ΪFeCl2������ȡ��������Ļ�ѧ����ʽΪ��FeS+ 2HCl==FeCl2+ H2S��
���������������װ��H������������Һ���������������кͷ�Ӧ����ѧ����ʽΪ��H2S + 2NaOH == Na2S + 2H2O��©�������������������������������

 �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�